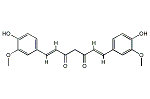
クルクミン
| 分子式: | C21H20O6 |
| その他の名称: | クルクミン、Curcumin、(1E,6E)-1,7-Bis(4-hydroxy-3-methylphenyl)-1,6-heptadiene-3,5-dione、(1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione、C.I.Natural Yellow 3、C.I.75300、C.I.ナチュラルイエロー3、ジフェルロイルメタン、Diferuloylmethane、trans,trans-クルクミン、trans,trans-Curcumin |
| 体系名: | (1E,6E)-1,7-ビス(4-ヒドロキシ-3-メトキシフェニル)-1,6-ヘプタジエン-3,5-ジオン |
クルクミン
クルクミン
出典: フリー百科事典『ウィキペディア(Wikipedia)』 (2025/04/30 21:05 UTC 版)
| クルクミン | |
|---|---|
 |
|
 |
|
|
(1E,6E)-1,7-bis (4-hydroxy- |
|
|
別称
curcumin
diferuloylmethane C.I. 75300 Natural Yellow 3 |
|
| 識別情報 | |
| CAS登録番号 | 458-37-7 |
| PubChem | 969516 |
| ChemSpider | 839564 |
| UNII | IT942ZTH98 |
| 日化辞番号 | J5.762B |
| E番号 | E100 (着色料) |
| KEGG | C10443 |
| ChEBI | |
| ChEMBL | CHEMBL116438 |
|
|
|
|
| 特性 | |
| 化学式 | C21H20O6 |
| モル質量 | 368.38 g/mol |
| 外観 | 鮮黄-橙色粉末 |
| 融点 | 183 °C (361 °F) (456.15 K) |
| 危険性 | |
| 無毒性量 NOAEL | ラット、経口、 720 mg/kg bw/day ヒト、経口、 8,000 mg/day [1] |
| 半数致死量 LD50 | マウス、経口、 2,000 mg/kg bw 以上 ラット、経口、 2,000 mg/kg bw 以上[1] |
| 特記なき場合、データは常温 (25 °C)・常圧 (100 kPa) におけるものである。 | |
クルクミン (英: curcumin) は、ウコン(ターメリック、学名: Curcuma longa)などに含まれる黄色のポリフェノール化合物[2]。クルクミノイドに分類される。スパイスや食品領域の着色剤として利用され[注 1]、日本ではウコン色素として既存添加物(着色料)に指定されている。[3][4]ウコン由来のクルクミンは「医薬品の範囲に関する基準」では医薬品でないものに分類され[2]、効用を謳わない限りは食品扱いとなる。
概要
鮮やかな黄色を持つことから、天然の食用色素として用いられる[2]。食用色素としての表示例としては、ウコン色素、クルクミン、ターメリック色素、などのように表記され、伝統的な用途例としては、漬物、水産ねり製品、栗のシロップ漬、和菓子などがあげられる。上掲写真のように鮮やかな黄色が有名であるが、酸性-中性条件の溶液中では明るい黄色を、塩基性(アルカリ性)条件では赤褐色(赤茶色)を呈す[5]。アルコール溶液(またはジエチルエーテル溶液)でも鮮黄色となるが、紫外線下では青緑色の蛍光を発する。
俗に、「抗酸化作用がある」「肝臓によい」「発がんを抑制する」などとされ[2]、様々なドリンク剤や健康食品(栄養補助食品)が販売されているが、人間での有効性・安全性に関して、信頼に足る立証はなされていない[2]。
ホウ酸と反応して赤色の化合物ロソシアニンを生じるため、ホウ素の定量に用いることができる(クルクミン法)[6]。 ケト型とエノール型の2つの互変異性体が存在し、固体および溶液中においては後者の方がエネルギー的に安定である[7]。フィトケミカル(植物が由来の化学物質で、植物が自分達の身体を守るために作り出す自己防衛成分-野菜の色素や辛味成分)に分類され、上記のようにポリフェノール類の一種でもあり、抗酸化物質としても知られている。
化学的構造


クルクミンはいくつかの官能基によって構成されており、ポリフェノールの芳香族環が、2つのα,β-不飽和カルボニル基で連結された構造をとっている。ジケトン体は安定したエノール型もしくは、α,β-不飽和カルボニル基中で容易に脱プロトン化したエノラートを形成し、マイケル反応の良い受容体になって求核付加を受ける。
この化学的構造は、1910年に、J. Miłobędzka、Stanisław KostaneckiおよびWiktor Lampeによって同定された[8]。
水に難溶性であり、可視光や、紫外光、弱アルカリ条件下で分解され易い性質を有するため[9][10]、利用できる範囲を拡大できるように、各種の改善手法(配糖化、補助剤添加、ナノミセル化、超細粒化、フィトソーム化など)が試みられることが多い。熱には比較的安定であるが、耐光性(特に紫外線)には弱く、微量の金属イオン(特に鉄イオン)の共存でも暗色化する傾向にある。
生合成について
商業ベースでのクルクミンは、伝統的な生薬原料の製作と同様に、栽培されたウコン等を原材料にし、有機溶媒抽出法やアルコール抽出法などによって、分離および抽出を行い、生産することが現在は主とされている(抽出によって得られたクルクミンは有機溶媒等の除去が必要となる場合がある)。また下記のように生合成の正確な経路の探索および生化学的な(酵素利用による)合成法の研究もさかんに検討されている。
生合成経路の探索
クルクミンの生合成経路を定義することは、これまで合成研究者にとって非常に困難であった。まず、1973年に、RoughlyとWhitingがクルクミンの生合成について2つの経路を提唱した。最初の合成経路は、ケイ皮酸(英: cinnamic acid;シンナミック酸)と5分子のマロニルCoAによる鎖伸長反応が含まれる(最終的にはアリール基と結合してクルクミノイドとなる)。第2の経路には、2つのシンナメート(ケイ皮酸エステル)をマロニルCoAによって会合する反応が含まれているものであった。
どちらの合成経路も出発物質として、ケイ皮酸(英: cinnamic acid;シンナミック酸)が用いられており、元来はアミノ酸のフェニルアラニンから(アンモニアが脱離することで)派生してくるものである。植物の生合成において、ケイ皮酸を出発物質として活用しているということは、より一般的なp-クマル酸の利用と比べると稀なことであり、注目に値する事項である。他にはわずかに、アニゴルホン (英: anigorufone)[11]およびピノシルビン (英: pinosylvin)[12]のみが少数例として、ケイ皮酸を出発物質として利用する。
また2008年までは実験的に確証のとれた合成経路は存在しておらず、このときの喜多[13]らの実験で仮定された生合成の経路もRoughlyとWhitingによって提唱された第1および第2の経路に従ったものであった。
しかしながら、13C同位体標識法によるデータは、5分子のマロニルCoAがケイ皮酸と反応してクルクミンを形成するという第1の経路を支持してはいたが、ヒドロキシ基やメトキシ基といった官能基がクルクミノイドに組み込まれていく順番が、第2の合成経路を強く支持するものであった。したがって、RoughlyとWhitingの提唱した経路のうち第2の経路が正しいことが結論づけられた。

生化学的な(酵素利用による)合成法の研究
従来の植物(栽培されたウコン等)からの抽出法、石油資源由来の炭素源を元にする化学合成法に加えて、他の植物ポリフェノールと同じように微生物による生産法の研究も進められている(上記の生合成経路の判明により、本格的な検討が可能となってきた)。微生物による生産は、従来法と比べて大量生産(スケールアップ)が可能であったり、出発基質の変更による改良された派生物質の生産、産業廃棄物(米糠)の有効利用などの点について、利点が見いだせると言われている。
クルクミンは、芳香族ポリケチド(ポリケタイド)に分類される。ここでいうポリケチドとは、アセチルCoAなどのCoAエステル(もしくはACP体)を出発基質とし、マロニルCoAを代表物質とする伸張鎖基質を伸張物質として、ポリケトン鎖を合成(縮合反応)した後、様々な修飾を受けて生合成される化合物の総称である(詳しくはポリケチドの項を参照)。代表的なポリケチドとしては、エリスロマイシン、ピクロマイシンなどのマクロライド系抗生物質、ラパマイシンなどのポリエン系抗生物質、芳香族ポリケチド(ポリフェノール)、ウバリシンなどの抗癌作用物質があげられる。
また、ポリケチドはポリケチド合成酵素(PKS)を触媒として生合成される。PKSにはI型、II型、III型の3種類が存在するが、III型PKSとは植物の中でポリケタイドという成分が作られる時に働く酵素の総称となる(植物からは、さまざまな種類のポリケタイドを作るさまざまな種類の酵素が見つかっており、構造や性質が似ているこれらの酵素をIII型PKSと総称している)。
III型PKSとしては、カルコン合成酵素(CHS)が代表的であるが、ウコン中でクルクミンが作られる時に働く酵素は、DCS(英: diketide CoA synthase;ジケチドCoA生合成酵素)と、CURS(英: curcumin synthease;クルクミン生合成酵素)の2種類の酵素が働いて、クルクミンを作っている可能性が高いことが報告されている(なお、通常のマロニルCoAを伸張物質とするIII型PKSと違い、CURSはマロニルCoAではない物質(フェルロイルCoA)を付加する珍しい酵素である)。
クルクミンを合成する酵素は、現在までにDCSで1つ、CURSで3つの遺伝子が単離されている。これは、当初、全ゲノム解析の行われていたイネゲノム中で、約30種類の機能未知なカルコン合成酵素様遺伝子を網羅的に解析していた過程で、クルクミノイド合成酵素として報告されてきた遺伝子である[14][15]。その情報を元にして、上記のウコンでの生合成経路の探索も進められていった。上記の知見で得られた酵素を利用し、工業微生物と安価な基質(産業廃棄物としての米糠)を用いた生産工程も研究されている。
医学的利用の可能性
クルクミンの生理作用として抗腫瘍作用や抗酸化作用、抗アミロイド作用、抗炎症作用などが知られている。
抗炎症作用はエイコサノイド合成の阻害によるものだと考えられている[16]。また、フリーラジカル捕捉能を持ち、脂質の過酸化や活性酸素種によるDNA傷害を防ぐ。クルクミノイドはグルタチオン-S-トランスフェラーゼを誘導するため、シトクロムP450を阻害しうる。
クルクミンの生理活性と医学的有用性は近年盛んに研究されている。抗がん効果では、がん細胞特異的にアポトーシスを誘導するとの報告がある。また、クルクミンはがんをはじめとした多くの炎症性疾患に関連する転写因子であるNF-κBを抑制しうる[17]。実際、事前に発がん物質を投与されたマウスやラットに、0.2%のクルクミンを添加した食餌を与えたところ、大腸癌の発症において有意な減少が見られたとの報告がある[18]。
2004年、カリフォルニア大学ロサンゼルス校 (UCLA) の研究チームはアルツハイマー病モデルマウスを用いて実験を行い、クルクミンが脳におけるβアミロイドの蓄積を抑制し、アミロイド斑を減少させることを示した[19]。
クルクミンが精神的機能に影響をおよぼすとの疫学的調査結果も存在する。高齢のアジア人を対象としたミニメンタルステート検査で、半年に1度以上黄色カレーを食する群において相対的に高いスコアが見られた[20]。ただし、科学的見地から見れば、この結果はカレー食がもたらしたものか、精神的に健康な人がカレーを好んで食べるのか判断できないし、これらとは全く異なる理由によるのかも知れない。
食事からはごく少量のクルクミンしか体内に吸収されないとの報告もある[21]。黒コショウ成分ピペリンと同時に摂取することで腸管吸収性の改善が見られるとの報告もあるが、この成分は薬物代謝に影響をおよぼすため、摂取には注意を要する。クルクミンに期待される有益な作用の中には、例えば大腸癌のリスク低減など、必ずしも腸管吸収を必要としないものもある。
クルクミンの蛍光特性を利用して、手術中病理組織診断へ応用する試みがある[22]。
クルクミンの生体吸収性改善の試みについて
2007年には、クルクミンの高分子ナノ粒子を用いたカプセル化製剤(ナノクルクミン "nanocurcumin")が製剤化された。この製剤は、フリー体(遊離体)のクルクミンに付き物の、難水性や低い生物学的利用率(バイオアベイラビリティー)といった欠点の多くを回避できる可能性が期待できる。
このナノクルクミン粒子は平均100 nm未満のサイズであり、ヒト癌細胞株モデルにおいて、フリー体のクルクミンと比べて優れた効果に相当する結果を示している[23]。しかしながら、このナノ粒子での実際のin vivoでの吸収はまだ示されていない。
2008年7月には前述のUCLAの神経学の研究者たちが、50回の臨床試験を重ねることで、脳内 (in vivo) で5 μM以上の濃度を得られる脂質化クルクミンの形成について結果を報告している[24]。
また一方では、2006年にクルクミンのバイオアベイラビリティー増加させる手法(大豆リン脂質との混合物を作成する簡易な手順を含む)が特許出願されている[25]。この手法を用いた場合、血漿中クルクミン濃度が、フリー体のクルクミンで約5倍、グルクロン酸抱合体では20倍増加しているとの報告がある(対照コントロールとしての等モル量の未製剤化クルクミンと比較すると、フリー体の場合、血漿中濃度が33.4 nM/6.5 nMとなり、グルクロン酸抱合体の場合、4420 nM/225 nMという実測値となった)[26]。
2010年には、食品用の高分子ナノミセルの封入システムにより、クルクミンの水への溶解性およびin vitroでの抗がん活性が向上することが示された。通常、風味等を封入する用途に用いられる疎水性の変性デンプンが、高分子ナノミセルを形成することが明らかになった。よって簡単な高速ホモジナイズ法を用いて、クルクミンを疎水性のコア(核側)の部分に充填し、クルクミンの可溶化を行うことができる。検証のためのHepG2細胞株を用いた細胞培養実験では抗がん活性の増幅が明らかになった。ただしバイオアベイラビリティーの比率の観点で見た、有効性のさらなる証明には、in vivoでの追加試験が求められる[27]。
日本でも、細粒化技術により生体への吸収性を改善したクルクミン製剤が開発されている。本製剤では血中濃度でのフリー体クルクミンでの約30倍の濃度増加を確認している(セラクルミンの項を参照)[28]。
キレート作用
クルクミンには鉄をキレートする作用があると考えられており[29]、実験動物にて肝臓や脾臓などの臓器から鉄を減らし酸化ストレスを低減させる作用が報告されている。[30][31] 一部の慢性肝臓病や癌[32]などいくつかの疾患では鉄の過剰蓄積とその酸化ストレスと関連がある場合が存在するとみられており、それらの予防などへの応用が期待される。
モノアミン酸化酵素阻害作用
クルクミンはMAO-AおよびMAO-Bの両方を阻害する[33]。
有効性
変形性関節症に対するメタアナリシスでは証拠の質は低いが、痛みの軽減に効果量0.8以上と大きな効果を示している[34]。
クルクミンが経口摂取で慢性のブドウ膜炎(ブドウ膜:虹彩・毛様体・脈絡膜の総称)の治療に有用である可能性を示す予備的な臨床知見があるが[35]、この現象についてはさらなる検証が必要である。
また、慢性的な前部ブドウ膜炎患者15名を対象にクルクミン375 mg×3回/日、12週間摂取させたところ、症状の改善が見られたという予備的な報告がある[36]。
クルクミンは抗炎症作用を呈する細胞毒性のない濃度においてDNAに対する酸化ストレスを誘導するとの報告がある。[37]
安全性・副作用
以上のように、健康に有益な作用の期待されるクルクミンであるが、クルクミンを主たる成分とする「うこん(春ウコン、秋ウコン、クスリウコンなど)」は、生薬や漢方薬の素材、食品、民間的な傷薬、および天然顔料として、主にインド、東南アジア、中国、琉球諸島などの諸地域で、古来より長く利用されている。その経験から鑑みるに、食品および天然色素としてのクルクミンの使用については恐らく安全であると考えられている[38]。
また、うこん茶等による肝障害例も報告されているが、秋ウコンの根茎は、クルクミン原体の他にもミネラル分(鉄分)が豊富に含まれているものがある。例えばC型慢性肝炎患者は、鉄過剰を起こしやすいことから鉄制限食療法が実施されるが、そのような場合には、うこん含有の鉄分が肝臓に過剰な影響を及ばすことがあり、注意が必要といわれている[39](なお精製されたクルクミン原体の場合には、含有ミネラルの問題は起こらない)。
また、ワーファリン(ワルファリン)等を服用している場合、クルクミンにも血小板凝集抑制作用が知られているので、薬理効果が増強される可能性があり、他のビタミンK含有食品と同様に食べ合わせや摂取量に留意する必要が生ずる[38]。なお、医薬品的効能効果を目指す場合の検証も、報告例が見受けられる。
安全性について、がん患者における第1相臨床試験の結果では、クルクミン8,000 mg/日、3ヶ月間の経口投与は安全であったとの報告がある[40]。
また、クルクミンは、第61回JECFA(2003年6月)において添加物としての再評価がなされ、NOELは250-320mg/kg bw、ADIは0-3 mg/kgbwとされた[41][42][43]
潜在的なリスクおよび副次的効果の側面も報告されている。例えば、抗癌効果とは全く逆の、発癌性を有するとの報告もなされている[44]。腫瘍抑制に働くp53経路に干渉する可能性がある[45]が、マウスやラットを用いた動物試験では、クルクミン摂取と腫瘍発生の関連性は証明されなかった[46]。クルクミンの投与により皮膚炎になった、不整脈が発生という報告もある[2]。また乾癬患者12名に投与したところ3名の症状が悪化したとされる[2]。
食品衛生分野での利用
食品衛生分野ではエタノールで溶解した0.1%クルクミン溶液が脂肪と反応し、その部分に紫外線を照射すると蛍光黄色を発することから、脂肪汚れに対する食器等の洗浄効果の確認検査に用いられる[47]。
脚注
注釈
出典
- ^ a b “HSDB: CURCUMIN”. TOXNET, National Library of Medicine (NLM). 2017年10月4日閲覧。
- ^ a b c d e f g クルクミン - 素材情報データベース<有効性情報>(国立健康・栄養研究所) 2016年8月3日閲覧
- ^ “既存添加物名簿収載品目リスト”. 日本食品化学研究振興財団 (2014年1月30日). 2017年11月6日閲覧。
- ^ “食品添加物関連情報 クルクミン”. 国立医薬品食品衛生研究所安全情報部. 2017年11月6日閲覧。
- ^ 平松, 茂樹 (2017). “「化学」の授業で色の変化を見る”. 化学と教育 65 (8): 396–399. doi:10.20665/kakyoshi.65.8_396.
- ^ Methods for the Chemical Analysis of Water and Wastes (MCAWW) (EPA/600/4-79/020)
- ^ Manolova, Yana; Deneva, Vera; Antonov, Liudmil; at al (2014). “The effect of the water on the curcumin tautomerism: A quantitative approach”. Spectrochimica Acta 132A (1): 815–820. doi:10.1016/j.saa.2014.05.096.
- ^ J. Miłobȩdzka, St. v. Kostanecki, V. Lampe (1910). “Zur Kenntnis des Curcumins”. Berichte der deutschen chemischen Gesellschaft 43 (2): 2163–2170. doi:10.1002/cber.191004302168.
- ^ 坂元(佐々木)史歩、佐藤 恭子、阿部 雅美、杉本 直樹、米谷 民雄 (1998). “天然着色料ウコン色素の成分分析とクルクミンの光安定性 Components of Turmeric Oleoresin Preparations and Photo-stability of Curcumin”. 日本食品化学学会誌 Jpn. J. Food Chem. 5: 57-63. NAID 110007367140.
- ^ Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK (1997). “Stability of curcumin in buffer solutions and characterization of its degradation products”. J. Pharm. Biomed. Anal. 15 (12): 1867-1876. doi:10.1016/S0731-7085(96)02024-9. PMID 9278892.
- ^ Schmitt, B.; Holscher, D.; Schneider, B. (2000). “Variability of phenylpropanoid precursors in the biosynthesis of phenylphenalenones in Anigozanthos preissii”. Phytochemistry 53 (3): 331–337. doi:10.1016/S0031-9422(99)00544-0. PMID 10703053.
- ^ Gehlert, R.; Schoeppner, A.; Kindl, H. (1990). “Stilbene synthase from seedlings of Pinus sylvestris: purification and induction in response to fungal infection”. Molecular Plant-Microbe Interactions 3: 444–449. doi:10.1094/MPMI-3-444.
- ^ Kita, T.; Imai, S.; Sawada, H.; Kumagai, H.; Seto, H. (2008). “The biosynthetic pathway of curcuminoid in turmeric (Curcuma longa) as revealed by 13C-labeled precursors”. Biosci. Biotechnol. Biochem. 72: 1789–1798. PMID 18603793.
- ^ Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. (2009). “Curcuminoid Biosynthesis by Two Type III Polyketide Synthases in the Herb Curcuma longa”. J. Biol. Chem. 284 (17): 11160–11170. doi:10.1074/jbc.M900070200. PMC 2670121. PMID 19258320.
- ^ Katsuyama, Y.; Kita, T.; Horinouchi, S. (2009). “Identification and characterization of multiple curcumin synthases from the herb Curcuma longa”. FEBS Lett. 583 (17): 2799-2803. doi:10.1016/j.febslet.2009.07.029. PMID 19622354.
- ^ Srivastava KC, Bordia A, Verma SK (1995). “Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets”. Prostaglandins Leukot. Essent. Fatty Acids 52 (4): 223-227. PMID 7784468.
- ^ Aggarwal BB, Shishodia S (2004). “Suppression of the nuclear factor-κB activation pathway by spice-derived phytochemicals: reasoning for seasoning”. Ann. N. Y. Acad. Sci. 1030: 434-441. doi:10.1196/annals.1329.054. PMID 15659827.
- ^ “Chemoprevention of Colorectal Cancer - Database of agents & diets ranked by efficacy: A systematic review of experimental studies (men, rats, mice)”. Chemoprevention Database. 2011年11月17日閲覧。
- ^ Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM (2005). “Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo”. J. Biol. Chem. 280 (7): 5892-5901. doi:10.1074/jbc.M404751200. PMID 15590663.
- ^ Ng TP, Chiam PC, Lee T, Chua HC, Lim L, Kua EH (2006). “Curry consumption and cognitive function in the elderly”. Am. J. Epidemiol. 164 (9): 898-906. doi:10.1093/aje/kwj267. PMID 16870699.
- ^ Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998). “Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers”. Planta Med. 64 (4): 353-356. doi:10.1055/s-2006-957450. PMID 9619120.
- ^ 難病治療にウコンのチカラ、三重大が手術中病理組織診断に新技術発見…がん治療への応用も期待(読売新聞2025年4月30日)
- ^ Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A (2007). “Polymeric nanoparticle-encapsulated curcumin ("nanocurcumin"): a novel strategy for human cancer therapy”. J. Nanobiotechnology 5: 3. doi:10.1186/1477-3155-5-3. PMC 1868037. PMID 17439648.
- ^ Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA (2008). “Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease”. J. Pharmacol. Exp. Ther. 326 (1): 196-208. doi:10.1124/jpet.108.137455. PMC 2527621. PMID 18417733.
- ^ EP 20060004820, Giori, Andrea (Viale Ortles, 12, 20139 Milano, IT), Franceschi, Federico (Viale Ortles, 12, 20139 Milano, IT), published 2007-09-26, assigned to INDENA S.p.A. (Viale Ortles, 12, 20139 Milano, IT)
- ^ Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ (2007). “Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine”. Cancer Chemother. Pharmacol. 60 (2): 171–177. doi:10.1007/s00280-006-0355-x. PMID 17051370.
- ^ Yu, Hailong; Huang, Qingrong (2010). “Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch”. Food Chemistry 119: 669-674. doi:10.1016/j.foodchem.2009.07.018.
- ^ Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T (2011). “Innovative preparation of curcumin for improved oral bioavailability”. Biol. Pharm. Bull. 34 (5): 660-665. doi:10.1248/bpb.34.660. PMID 21532153.
- ^ “Iron chelation in the biological activity of curcumin.”. Free Radical Biology and Medicine 40 (7). (2006). doi:10.1016/j.freeradbiomed.2005.11.003. PMID 16545682.
- ^ “Curcumin may impair iron status when fed to mice for six months”. Redox Biology 2. (2014). doi:10.1016/j.redox.2014.01.018. PMID 24634837.
- ^ “Curcumin Attenuates Iron Accumulation and Oxidative Stress in the Liver and Spleen of Chronic Iron-Overloaded Rats”. PLoS One 10 (7): e0134156. (2015). doi:10.1371/journal.pone.0134156. PMID 26230491.
- ^ “Iron and cancer: more ore to be mined”. Nature Reviews Cancer 13: p.342. (2013). doi:10.1038/nrc3495.
- ^ Kulkarni, SK; Dhir, A (2010). “An overview of curcumin in neurological disorders”. Indian Journal of Pharmaceutical Sciences 72 (2): 149. doi:10.4103/0250-474X.65012. PMC 2929771. PMID 20838516.
- ^ Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ (February 2018). “Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis”. Br J Sports Med 52 (3): 167–175. doi:10.1136/bjsports-2016-097333. PMID 29018060.
- ^ Pharmacist’s Letter/Prescriber’s letter, ed (2006). Natural Medicine Comprehensive Database. ISBN 978-0978820510
- ^ Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P, Srimal RC (1999). “Efficacy of curcumin in the management of chronic anterior uveitis”. Phytother. Res. 13 (4): 318-322. PMID 10404539.
- ^ “Curcumin protects against cytotoxic and inflammatory effects of quartz particles but causes oxidative DNA damage in a rat lung epithelial cell line”. Toxicology and applied pharmacology 227 (1). (2008). doi:10.1016/j.taap.2007.10.002. PMID 18001810.
- ^ a b NIH (2011年5月6日). “MedlinePlus Supplements, Turmeric”. Natural Medicine Comprehensive Database Consumer Version. 2011年11月17日閲覧。
- ^ Iwata K, Iwasa M, Hara N, Matsumoto A, Kobayashi Y, Watanabe S, Adachi Y, Kaito M (2006). “Iron content and consumption of health foods by patients with chronic hepatitis C”. J. Gastroenterol. 41 (9): 919-920. doi:10.1007/s00535-006-1860-8. PMID 17048058.
- ^ Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001). “Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions”. Anticancer Res. 21 (4B): 2895-2900. PMID 11712783.
- ^ WHO (2004年). “Evaluation of certain food additives and contaminants (Sixty-first report of the Joint FAO/WHO Expert Committee on Food Additives” (pdf). WHO Technical Report Series, No. 922. 2011年11月17日閲覧。
- ^ CURCUMIN (html). WHO FOOD ADDITIVES SERIES: 52 (Report). JECFA.
- ^ CURCUMIN (html). Summary of Evaluations (Report). JECFA.
- ^ Kawanishi, S. Oikawa, S. Murata, M. (2005). “Evaluation for safety of antioxidant chemopreventive agents”. Antioxid. Redox Signal. 7 (11-12): 1728-1739. doi:10.1089/ars.2005.7.1728. PMID 16356133.
- ^ Moos PJ, Edes K, Mullally JE, Fitzpatrick FA (2004). “Curcumin impairs tumor suppressor p53 function in colon cancer cells”. Carcinogenesis 25 (9): 1611-1617. doi:10.1093/carcin/bgh163. PMID 15090465.
- ^ Lois Swirsky Gold (2007年10月3日). “Turmeric (>98% curcurmin)”. Carcinogenic Potency Database Project. 2011年11月17日閲覧。
- ^ “3. 食器等の洗浄効果の確認検査”. 文部科学省. 2020年6月5日閲覧。
参考文献
- Campbell, Frederick C.; Collett, Gavin P. (2005). “Chemopreventive properties of curcumin”. Future Oncol. 1 (3): 405-414. doi:10.1517/14796694.1.3.405. PMID 16556014.
- Ringman, John M.; Frautschy, Sally A.; Cole, Gregory M.; Masterman, Donna L.; Cummings, Jeffrey L.. “A potential role of the curry spice curcumin in Alzheimer's disease”. Curr. Alzheimer Res. 2 (2): 131-136. PMC 1702408. PMID 15974909.
- Aggarwal, Bharat B.; Kumar, Anushree; Aggarwal, Manoj S.; Shishodia, Shishir (2005). “Curcumin derived from turmeric (Curcuma longa): A spice for all seasons”. In Debasis Bagchi, Harry G. Preuss. Phytopharmaceuticals in Cancer Chemoprevention. CRC series in modern nutrition science Vol.2. CRC Press. pp. 349-387. ISBN 9780849315602
- Chattopadhyay, Ishita; Biswas, Kaushik; Bandyopadhyay, Uday; Banerjee, Ranajit K (2004). “Turmeric and curcumin: biological actions and medicinal applications”. Current Science 87 (1): 44-53.
- “ウコンから記憶力活性物質、アルツハイマー予防に期待”. 読売新聞. (2008年8月19日). オリジナルの2008年9月14日時点におけるアーカイブ。 2011年11月19日閲覧。
関連項目
外部リンク
- クルクミン - (オレゴン州大学・ライナス・ポーリング研究所)
- クルクミン - 素材情報データベース<有効性情報>(国立健康・栄養研究所)
- Turmeric and curcumin, from (M.D. Anderson Cancer Center)
- Turmeric and curcumin, from (Memorial Sloan-Kettering Cancer Center)
- Turmeric, from (University of Maryland Medical Center)
- 健康食品ナビ-東京都 (東京都福祉保健局-Tokyo Metropolitan Government Bureau of SocialWelfare and Public Health)
- ウコン色素(横浜市衛生研究所 - 食品衛生情報)
固有名詞の分類
- クルクミンのページへのリンク