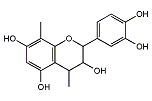
プロアントシアニジン
| 分子式: | C17H18O6 |
| その他の名称: | 2-(3,4-Dihydroxyphenyl)-4,8-dimethyl-3,4-dihydro-2H-1-benzopyran-3,5,7-triol、ミモザタンニン、Mimosatannin、2-(3,4-Dihydroxyphenyl)-3,4-dihydro-4,8-dimethyl-2H-1-benzopyran-3,5,7-triol、プロアントシアニジン、Proanthocyanidin |
| 体系名: | 2-(3,4-ジヒドロキシフェニル)-4,8-ジメチル-3,4-ジヒドロ-2H-1-ベンゾピラン-3,5,7-トリオール、2-(3,4-ジヒドロキシフェニル)-3,4-ジヒドロ-4,8-ジメチル-2H-1-ベンゾピラン-3,5,7-トリオール |
プロアントシアニジン
出典: フリー百科事典『ウィキペディア(Wikipedia)』 (2023/10/15 14:54 UTC 版)
プロアントシアニジン(英:proanthocyanidin)は、様々な植物に含まれるポリフェノールの一種である。プロアントシアニジンは、植物界において植物の葉、果実、樹皮、材などに広く分布しており、非常に多くの種類が存在する。フラバン-3-オール(flavan-3-ol)のフラバン骨格の炭素同士がC-C結合などによって結合したものであり、塩酸のような無機酸で加熱することによってC-Cなどの結合が切れてアントシアニジン(anthocyanidin)を生じるものとして知られている。生化学的には、多種多様な植物から得られるプロアントシアニジンの研究において、さまざまな生理活性を有することが報告されている。
- ^ Hayashi, K. The chemistry of flavonoid compounds. Edited by T.A.Geissman, Chapter 9 The anthocyanins. 248-285, 1962.
- ^ Willsttter, R.; Everest, A.E. Untersuchungen uber die anthocyane; Ⅰ. Uber den farbstoff der kornblume. Justus liebigs annalen der chemie. 401, 189-232, 1914.
- ^ Rosenheim, O. XXI. Observations on anthocyanins. I. The anthocyanins of the young leaves of the grape vine. Biochem. J. 14, 178-188, 1920.
- ^ Robinson, G.M.; Robinson, R. XXXI. A survey of anthocyanins. Ⅲ. Notes on the distribution of leuco-anthocyanins. Biochem. J., 27, 206-212, 1933.
- ^ Freudenberg, K.; Weinges, K. Systematik und nomenklatur der flavonoide. Tetraheron, 8, 334-349, 1960.
- ^ Freudenberg, K.; Weinges, K. The chemistry of flavonoid compounds. Edited by T.A.Geissman, Chapter 7 Catechins and flavonoid tannins, 197-216, 1962.
- ^ Weinges, K.; Freudenberg, K. Condensed proanthocyanidins from cranberries and cola nuts. chemical communications, 1965.
- ^ 武田幸作; 齋藤規夫; 岩科司編. 植物色素フラボノイド, p25, 2013.
- ^ a b Kusano, R.; Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y.; Kouno, I. α-Amylase and lipase inhibitory activity and structural characterization of Acacia bark proantocyanidins. J. Nat. Prod. 74, 119–128, 2011. doi: 10.1021/np100372t
- ^ María, Luisa, Mateos-Martín; Elisabet, Fuguet; Carmen, Quero; Jara, Pérez-Jiménez; Josep, Lluís, Torres; Fuguet; Quero; Pérez-Jiménez; Torres. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Analytical and Bioanalytical Chemistry. 402, 1327–1336, 2012. doi:10.1007/s00216-011-5557-3 PMID 22101466.
- ^ Souquet, J; Cheynier, Véronique; Brossaud, Franck; Moutounet, Michel Polymeric proanthocyanidins from grape skins. Phytochemistry. 43, 509–512, 1996. doi:10.1016/0031-9422(96)00301-9
- ^ "USDA Database for the Proanthocyanidin Content of Selected Foods – 2004" (PDF). USDA. 2004. Retrieved 24 April 2014.
- ^ María, Luisa, Mateos-Martín; Elisabet, Fuguet; Carmen, Quero; Jara, Pérez-Jiménez; Josep, Lluís, Torres; Fuguet; Quero; Pérez-Jiménez; Torres. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Analytical and Bioanalytical Chemistry. 402, 1327–1336, 2012. doi:10.1007/s00216-011-5557-3 PMID 22101466.
- ^ Carpenter J.L., Caruso F.L., Tata A., Vorsa N., Neto C.C. Variation in proanthocyanidin content and composition among commonly grown North American cranberry cultivars (Vaccinium macrocarpon). J Sci Food Agric. 94, 2738–2745, 2014. doi:10.1002/jsfa.6618
- ^ Taheri, Rod; Connolly, Bryan A.; Brand, Mark H.; Bolling, Bradley W. Underutilized Chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) Accessions Are Rich Sources of Anthocyanins, Flavonoids, Hydroxycinnamic Acids, and Proanthocyanidins. Journal of Agricultural and Food Chemistry. 61, 8581–8588, 2013. doi:10.1021/jf402449qPMID 23941506.
- ^ Rösch, Daniel R.; Mügge, Clemens; Fogliano, Vincenzo; Kroh, Lothar W. (2004). "Antioxidant Oligomeric Proanthocyanidins from Sea Buckthorn (Hippophaë rhamnoides) Pomace". Journal of Agricultural and Food Chemistry. 52, 6712–6718, 2004. doi:10.1021/jf040241g
- ^ “Concentrations of proanthocyanidins in common foods and estimations of normal consumption”. The Journal of nutrition 134 (3). (2004). doi:10.1093/jn/134.3.613. PMID 14988456.
- ^ Roux, D.G. Study of the affinity of black wattle extract constituents. Part I. Affinity of polyphenols for swollen collagen and cellulose in water. J. Soc. Leather Trades’ Chem. 39, 80–91, 1955.
- ^ Botha, J.J.; Ferreira, D.; Roux, D.G. Condensed Tannins: Direct Synthesis, Structure, and Absolute. Configuration of Four Biflavonoids from Black Wattle Bark (‘Mimosa’) Extract. J. Chem. Soc. Chem. Commun. 700–702, 1978.
- ^ Yazaki, Y. Utilization of Flavonoid Compounds from Bark andWood: A Review. Nat. Prod. Commun. 10, 513-520, 2015.
- ^ Liu, X.; Wang, F. Investigation on biological activities of proanthocyanidins from black wattle bark. Chem. Ind. For. Prod. 27, 43–48, 2007.
- ^ Ohara, S.; Suzuki, K.; Ohira, T. Condensed tannins from Acacia mearnsii and their biological activities. Mokuzai Gakkaishi. 40, 1363–1374, 1994.
- ^ Olajuyigbe, O.O.; Afolayan, A.J. In vitro antibacterial and time-kill assessment of crude methanolic stem bark extract of Acacia mearnsii DeWild against bacteria in shigellosis. Molecules 17, 2103–2118, 2012.
- ^ Olajuyigbe, O.O.; Afolayan, A.J. A comparative effect of the alcoholic and aqueous extracts of Acacia mearnsii DeWild on protein leakage, lipid leakage and ultrastructural changes in some selected bacterial strains as possible mechanisms of antibacterial action. J. Pure Appl. Microbiol. 8, 1243–1257, 2014.
- ^ a b 9
- ^ Ikarashi, N.; Takeda, R.; Ito, K.; Ochiai, W.; Sugiyama, K. The Inhibition of Lipase and Glucosidase Activities by Acacia Polyphenol. Evid.-Based Complement. Alternat. Med. 2011, 2011. 272075. doi: 10.1093/ecam/neq043
- ^ Matsuo, Y.; Kusano, R.; Ogawa, S.; Yazaki, Y.; Tanaka, T. Characterization of the a-Amylase Inhibitory Activity of Oligomeric Proanthocyanidin from Acacia mearnsii Bark Extract. Nat. Prod. Commun. 11, 1851–1854, 2016.
- ^ 23
- ^ Ikarashi, N.; Toda, T.; Okaniwa, T.; Ito, K.; Ochiai, W.; Sugiyama, K. Anti-obesity and anti-diabetic effects of Acacia polyphenol in obese diabetic KKAy mice fed high-fat diet. Evid.-Based Complement. Alternat. Med. 2011, 2011. 952031. doi: 10.1093/ecam/nep241
- ^ 片岡武司; 小川壮介; 松前智之; 矢崎義和; 山口英世. アカシアポリフェノール含有食品の安全性:健常男性成人における安全性評価試験 応用薬理 80, 43-52, 2011.
- ^ Ogawa, S.; Miura, N. Safety Evaluation Study on Overdose of Acacia Bark Extract (Acacia Polyphenol) in Humans—A Randomized, Double-blind, Placebo-controlled, Parallel Study. Jpn. Pharmacol. Ther. 45, 1927–1934. 2017.
- ^ Ogawa, S.; Matsumae, T.; Kataoka, T.; Yazaki, Y.; Yamaguchi, H. Effect of acacia polyphenol on glucose homeostasis in subjects with impaired glucose tolerance:A randomized multicenter feeding trial. Experimental and therapeutic medicine 5, 1566-1572, 2013. doi: 10.3892/etm.2013.1029
- ^ Takeda, R.; Ogawa, S.; Miura, N.; Sawabe, A. Suppressive Effect of Acacia Polyphenol on Postprandial Blood Glucose Elevation in Non-diabetic Individuals—A Randomized, Double-blind, Placebo-controlled Crossover Study. Jpn. Pharmacol. Ther. 44, 1463–1469, 2016.
- ^ Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y. Utilization of Flavonoid Compounds from Bark and Wood. Ⅲ. Application in Health Foods. molecules 23, 1860, 2018. Utilization of Flavonoid Compounds from Bark and Wood. III. Application in Health Foods
- ^ Saito, M.; Hosoyama, H.; Ariga, T.; Kataoka, S.; Yamaji, N. Antiulcer activity of grape seed extract and procyanidins J. Agric. Food Chem., 46, 1460-1464, 1998. Utilization of Flavonoid Compounds from Bark and Wood. III. Application in Health Foods
- ^ Jun ,Yamakoshi; Shigehiro, Kataoka; Takuro, Koga; Toshiaki, Ariga. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. 142, 139-149, 1999. doi:10.1016/S0021-9150(98)00230-5
- ^ K. Karthikeyan; B.R. Sarala Bai; S. Niranjali Devaraj. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. International Journal of Cardiology, 115, 326-333, 2007. doi:10.1016/j.ijcard.2006.03.016
- ^ Debasis, Bagchi; Anand, Swaroop; Harry, G. Preuss, Manashi Bagchi. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 768, 69-73, 2014, doi:10.1016/j.mrfmmm.2014.04.004
- ^ 有賀敏明 プロアントシアニジンの抗酸化機能および疾病予防機能とその応用, 日本油化学会誌, 48, 1999.
- ^ Steigerwalt, Robert; Belcaro, Gianni; Cesarone, Maria, Rosaria; Di Renzo, Andrea; Grossi, Maria, Giovanna; Ricci, Andrea; Dugall, Mark; Cacchio, Marisa; Schönlau, Frank Pycnogenol Improves Microcirculation, Retinal Edema, and Visual Acuity in Early Diabetic Retinopathy. Journal of Ocular Pharmacology and Therapeutics. 25, 537–540, 2009.
- ^ Schoonees, A; Visser, J; Musekiwa, A; Volmink, J. Pycnogenol® (extract of French maritime pine bark) for the treatment of chronic disorders. The Cochrane Database of Systematic Reviews (4) 2012. doi:10.1002/14651858.CD008294pub4. P.
- ^ Corder, R.; Mullen, W.; Khan, N. Q.; Marks, S. C.; Wood, E. G.; Carrier, M. J.; Crozier, A. Oenology: Red wine procyanidins and vascular health. Nature. 444 (7119), 566, 2006. doi:10.1038/444566a PMID 17136085.
- ^ Absalon, C; Fabre, S; Tarascou, I; Fouquet, E; Pianet, New strategies to study the chemical nature of wine oligomeric procyanidins. Analytical and Bioanalytical Chemistry. 401, 1485–1495, 2011. doi:10.1007/s00216-011-4988-1 PMID 21573848.
- ^ Gonzalo-Diago, A; Dizy, M; Fernández-Zurbano, P Taste and mouthfeel properties of red wines proanthocyanidins and their relation to the chemical composition". Journal of Agricultural and Food Chemistry. 61, 8861–8870, 2013. doi:10.1021/jf401041
- ^ Sánchez-Moreno, Concepción; Cao, Guohua; Ou, Boxin; Prior, Ronald L. Anthocyanin and Proanthocyanidin Content in Selected White and Red Wines. Oxygen Radical Absorbance Capacity Comparison with Nontraditional Wines Obtained from Highbush Blueberry. Journal of Agricultural and Food Chemistry. 51, 4889–4896, 2003. doi:10.1021/jf030081tPMID 12903941.
- ^ Stringano, E; Gea, A; Salminen, J. P.; Mueller-Harvey, I. Simple solution for a complex problem: Proanthocyanidins, galloyl glucoses and ellagitannins fit on a single calibration curve in high performance-gel permeation chromatography. Journal of Chromatography A. 1218, 7804–7812, 2011. doi:10.1016/j.chroma.2011.08.082
- ^ Engström, M. T.; Pälijärvi, M; Fryganas, C; Grabber, J. H.; Mueller-Harvey, I; Salminen, J. P. Rapid Qualitative and Quantitative Analyses of Proanthocyanidin Oligomers and Polymers by UPLC-MS/MS". Journal of Agricultural and Food Chemistry. 62, 3390–3399, 2014. doi:10.1021/jf500745y
- 1 プロアントシアニジンとは
- 2 プロアントシアニジンの概要
- 3 代表的なプロアントシアニジンの生理活性
- 4 化学
- プロアントシアニジンのページへのリンク