The Organogermanium Compound THGP Suppresses Melanin Synthesis via Complex Formation with L-DOPA on Mushroom Tyrosinase and in B16 4A5 Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. THGP and L-DOPA Form a Complex via a Cis-Diol Structure
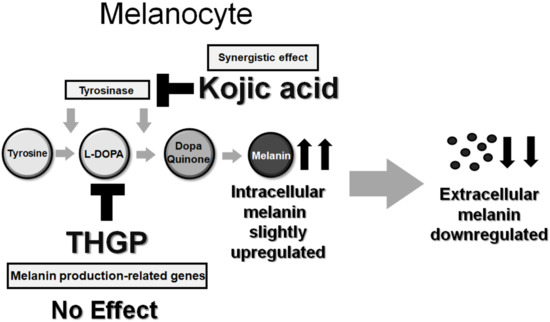
2.2. THGP Inhibits Melanin Synthesis through a Reaction Mediated by Mushroom-Derived Tyrosinase
2.3. THGP Inhibits Melanin Production by the B16 4A5 Melanoma Cell Line
2.4. THGP Does Not Affect Tyrosinase Activity or the Expression of Melanin Synthesis-Related Genes
2.5. THGP Suppresses Melanin Production in α-MSH- or L-DOPA-Induced Melanogenesis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. 1H-NMR
4.4. Enzymatic Reaction with Mushroom Tyrosinase
4.5. MTS Assay
4.6. Measurement of the Melanin Content in Cells
4.7. Measurement of Tyrosinase Activity
4.8. Quantitative Polymerase Chain Reaction (PCR)
4.9. Measurement of the Melanin Content in Cells Treated with α-MSH
4.10. Measurement of the Melanin Content in Cells Treated With L-DOPA
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ballato, J.; Hawkins, T.; Foy, P.; McMillen, C.; Morris, S.; Stolen, R.; Rice, R. Advances in Semiconductor Core Optical Fiber. In Proceedings of the IEEE Winter Topicals 2011, Keystone, CO, USA, 10–12 January 2011; pp. 191–192. [Google Scholar]
- Tsutsui, M.; Kakimoto, N.; Axtell, D.; Oikawa, H.; Asai, K. ChemInform Abstract: Crystal structure of ′carboxyethylgermanium sesquioxide. Chemischer Informationsdienst 1977. [Google Scholar] [CrossRef]
- Tsutsui, M.; Kakimoto, N.; Axtell, D.D.; Oikawa, H.; Asai, K. Crystal structure of “carboxyethylgermanium sesquioxide”. J. Am. Chem. Soc. 1976, 98, 8287–8289. [Google Scholar] [CrossRef]
- Nakamura, T.; Saito, M.; Aso, H. Effects of a lactobacilli, oligosaccharide and organic germanium intake on the immune responses of mice. Biosci. Biotechnol. Biochem. 2012, 76, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Takeda, T.; Tokuji, Y. The Oral Intake of Organic Germanium, Ge-132, Elevates α-Tocopherol Levels in the Plas-ma and Modulates Hepatic Gene Expression Profiles to Promote Immune Activation in Mice. Int. J. Vitam. Nutr. Res. 2014, 84, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Aso, H.; Shibuya, E.; Suzuki, F.; Nakamura, T.; Inoue, H.; Ebina, T.; Ishida, N. Antitumor effect in mice of an organic germanium compound (Ge-132) when different administration methods are used. Gan Kagaku Ryoho. Cancer Chemother. 1985, 12, 2345–2351. [Google Scholar]
- Dozono, H.; Ikeda, K.; Onishi, T. Effectiveness of Ge-132 to relieve pain and smooth home care administration for the terminal cancer patient. Gan To Kagaku Ryoho 1996, 23, 291–295. [Google Scholar]
- Suzuki, F.; Brutkiewicz, R.R.; Pollard, R.B. Ability of sera from mice treated with Ge-132, an organic germanium compound, to inhibit experimental murine ascites tumours. Br. J. Cancer 1985, 52, 757. [Google Scholar] [CrossRef] [PubMed]
- Miyao, K. Toxicology and phase I studies on a novel organogermanium compound, Ge-132. Curr. Chemother. Infec. Dis. 1979, 2, 1527–1529. [Google Scholar]
- Sugiya, Y.; Sakamaki, S.; Sugita, T.; Abo, Y.; Sato, H. Subacute oral toxicity of carboxyethylgermanium sesquioxide (Ge-132) in rats. Ouyou Yakuri 1986, 31, 1181–1190. [Google Scholar]
- Iwadate, K.; Yamaguchi, Y.; Sasaki, M.; Nakatani, M.; Doi, Y.; Imai, N.; Tamano, S.; Nishihori, Y. Carcinogenicity study of poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) in F344 rats. Fundam. Toxicol. Sci. 2018, 5, 127–140. [Google Scholar] [CrossRef]
- Doi, Y.; Imai, N.; Suguro, M.; Numano, T.; Furukawa, F. No carcinogenicity of poly-trans-[(2-carboxyethyl) germasesquioxane] (Ge-132): 26-week feeding study using rasH2 mice. Fundam. Toxicol. Sci. 2017, 4, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Shimada, Y.; Takeda, T.; Sato, K.; Akiba, M.; Fukaya, H. Organogermanium compound, Ge-132, forms complexes with adrenaline, ATP and other physiological cis-diol compounds. Future Med. Chem. 2015, 7, 1233–1246. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, K.; Takeda, T.; Tokuji, Y. The Organogermanium Compound Ge-132 Interacts with Nucleic Acid Components and Inhibits the Catalysis of Adenosine Substrate by Adenosine Deaminase. Biol. Trace Elem. Res. 2018, 181, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mosby, N.; Yang, J.; Xu, A.; Abdel-Malek, Z.; Kadekaro, A. α-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009, 22, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hearing, V.J. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev. Derm. 2011, 6, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Jung, H.; Lee, J.; Oh, H.; Kim, O.; Han, I.-O.; Oh, E.S. Keratinocyte-derived Laminin-332 Protein Promotes Melanin Synthesis via Regulation of Tyrosine Uptake. J. Biol. Chem. 2014, 289, 21751–21759. [Google Scholar] [CrossRef] [Green Version]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef]
- Marmol, V.; Ito, S.; Jackson, I.; Vachtenheim, J.; Berr, P.; Ghanem, G.; Morandini, R.; Wakamatsu, K.; Huez, G. TRP-1 expression correlates with eumelanogenesis in human pigment cells in culture. FEBS Lett. 1993, 327, 307–310. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharm. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [PubMed]
- Funayama, M.; Arakawa, H.; Yamamoto, R.; Nishino, T.; Shin, T.; Murao, S. Effects of alpha- and beta-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci. Biotechnol. Biochem. 1995, 59, 143–144. [Google Scholar] [CrossRef]
- Hu, F.; Lesney, P.F. The isolation and cytology of two pigment cell strains from B16 mouse melanomas. Cancer Res. 1964, 24, 1634–1643. [Google Scholar]
- Shimada, Y.; Sato, K.; Tokuji, Y.; Nakamura, T. Nuclear magnetic resonance studies of the interactions between the organic germanium compound Ge-132 and saccharides. Carbohyd. Res. 2015, 407, 10–15. [Google Scholar] [CrossRef]
- Nagasawa, T.; Sato, K.; Shimada, Y.; Kasumi, T. Efficient Conversion of D-Glucose to D-Fructose in the Presence of Organogermanium Compounds. J. Appl. Glycosci. 2016, 63, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.H.; Lin, T.; Wang, Z.T.; Wei, D.Z.; Xiang, H.B. Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J. Enzym. Inhib. Med. Chem. 2007, 22, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Derm. Sci 2015, 80, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratford, M.; Ramsden, C.A.; Riley, P.A. The influence of hydroquinone on tyrosinase kinetics. Bioorganic Med. Chem. 2012, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells. Chem. Biol. Interact. 2008, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Karačonji, I.B.; Benković, V.; Kopjar, N. In vitro assessment of the cytotoxic, DNA damaging, and cytogenetic effects of hydroquinone in human peripheral blood lymphocytes. Arh Hig Rada Toksikol 2017, 68, 322–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Baek, N.; Nam, T. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jeon, Y.; Kim, D.Y.; Lee, E.; Hyun, S.-H.H. Antioxidative effect of carboxyethylgermanium sesquioxide (Ge-132) on IVM of porcine oocytes and subsequent embryonic development after parthenogenetic activation and IVF. Theriogenology 2015, 84, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hwang, S.-U.U.; Yoon, J.D.; Jeung, E.-B.B.; Lee, E.; Kim, D.Y.; Hyun, S.-H.H. Carboxyethylgermanium sesquioxide (Ge-132) treatment during in vitro culture protects fertilized porcine embryos against oxidative stress induced apoptosis. J. Reprod. Dev. 2017, 63, 581–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Hanyu, T.; Nozaki, K.; Kataoka, K.; Kawatani, T.; Asahi, T.; Sawamura, N. Antioxidant Activity of Ge-132, a Synthetic Organic Germanium, on Cultured Mammalian Cells. Biol. Pharm. Bull. 2018, 41, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutkiewicz, R.; Suzuki, F. Biological activities and antitumor mechanism of an immunopotentiating organogermanium compound, Ge-132 (review). In Vivo 1987, 1, 189–203. [Google Scholar] [PubMed]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Derm. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed]








| Forward | Reverse | |
|---|---|---|
| Tyrosinase | TTGCCACTTCATGTCATCATAGAATATT | TTTATCAAAGGTGTGACTGCTATACAAAT |
| Trp-1 | ATGCGGTCTTTGACGAATGG | CGTTTTCCAACGGGAAGGT |
| Trp-2 | CTCAGAGCTCGGGCTCAGTT | TGTTCAGCACGCCATCCA |
| Mitf | CGCCTGATCTGGTGAATCG | CCTGGCTGCAGTTCTCAAGAA |
| Actb | CTAAGGCCAACCGTGAAAAG | ACCAGAGGCATACAGGGACA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azumi, J.; Takeda, T.; Shimada, Y.; Aso, H.; Nakamura, T. The Organogermanium Compound THGP Suppresses Melanin Synthesis via Complex Formation with L-DOPA on Mushroom Tyrosinase and in B16 4A5 Melanoma Cells. Int. J. Mol. Sci. 2019, 20, 4785. https://doi.org/10.3390/ijms20194785
Azumi J, Takeda T, Shimada Y, Aso H, Nakamura T. The Organogermanium Compound THGP Suppresses Melanin Synthesis via Complex Formation with L-DOPA on Mushroom Tyrosinase and in B16 4A5 Melanoma Cells. International Journal of Molecular Sciences. 2019; 20(19):4785. https://doi.org/10.3390/ijms20194785
Chicago/Turabian StyleAzumi, Junya, Tomoya Takeda, Yasuhiro Shimada, Hisashi Aso, and Takashi Nakamura. 2019. "The Organogermanium Compound THGP Suppresses Melanin Synthesis via Complex Formation with L-DOPA on Mushroom Tyrosinase and in B16 4A5 Melanoma Cells" International Journal of Molecular Sciences 20, no. 19: 4785. https://doi.org/10.3390/ijms20194785