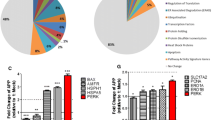
Abstract
Lafora disease (LD, OMIM 254780, ORPHA501) is a fatal neurodegenerative disorder characterized by the presence of glycogen-like intracellular inclusions called Lafora bodies and caused, in the vast majority of cases, by mutations in either EPM2A or EPM2B genes, encoding respectively laforin and malin. In the last years, several reports have revealed molecular details of these two proteins and have identified several processes affected in LD, but the pathophysiology of the disease still remains largely unknown. Since autophagy impairment has been reported as a characteristic treat in both Lafora disease cell and animal models, and as there is a link between autophagy and mitochondrial performance, we sought to determine if mitochondrial function could be altered in those models. Using fibroblasts from LD patients, deficient in laforin or malin, we found mitochondrial alterations, oxidative stress and a deficiency in antioxidant enzymes involved in the detoxification of reactive oxygen species (ROS). Similar results were obtained in brain tissue samples from transgenic mice deficient in either the EPM2A or EPM2B genes. Furthermore, in a proteomic analysis of brain tissue obtained from Epm2b−/−mice, we observed an increase in a modified form of peroxiredoxin-6, an antioxidant enzyme involved in other neurological pathologies, thus corroborating an alteration of the redox condition. These data support that oxidative stress produced by an increase in ROS production and an impairment of the antioxidant enzyme response to this stress play an important role in development of LD.










Similar content being viewed by others
Abbreviations
- CCCP :
-
carbonyl cyanide m-chlorophenylhydrazone
- ER :
-
endoplasmic reticulum
- LBs :
-
Lafora bodies
- LD :
-
Lafora disease
- 2D-DIGE :
-
two-dimensional differential in gel electrophoresis
- Prdx6 :
-
peroxiredoxin-6
- ROS :
-
reactive oxygen species
- VDAC2 :
-
voltage-dependent anion-selective channel protein 2
References
Lafora GR, Glueck BZ (1911) Beitrag zur Histopathologie der myoklonischen Epilepsie. Ges Neurol Psychiat 6:1–14
Lohi H, Ianzano L, Zhao XC, Chan EM, Turnbull J, Scherer SW, Ackerley CA, Minassian BA (2005) Novel glycogen synthase kinase 3 and ubiquitination pathways in progressive myoclonus epilepsy. Hum Mol Genet 14:2727–2736. doi:10.1093/hmg/ddi306
Rubio-Villena C, García-Gimeno MA, Sanz P (2013) Glycogenic activity of R6, a protein phosphatase 1 regulatory subunit, is modulated by the laforin–malin complex. Int J Biochem Cell Biol 45:1479–1488. doi:10.1016/j.biocel.2013.04.019
Worby CA, Gentry MS, Dixon JE (2008) Malin decreases glycogen accumulation by promoting the degradation of protein targeting to glycogen (PTG). J Biol Chem 283:4069–4076. doi:10.1074/jbc.M708712200
Solaz-Fuster MC, Gimeno-Alcaniz JV, Ros S, Fernandez-Sanchez ME, Garcia-Fojeda B, Criado Garcia O, Vilchez D, Dominguez J, Garcia-Rocha M, Sanchez-Piris M, Aguado C, Knecht E, Serratosa J, Guinovart JJ, Sanz P, Rodriguez de Cordoba S (2008) Regulation of glycogen synthesis by the laforin–malin complex is modulated by the AMP-activated protein kinase pathway. Hum Mol Genet 17:667–678. doi:10.1093/hmg/ddm339
Wang J, Stuckey JA, Wishart MJ, Dixon JE (2002) A unique carbohydrate binding domain targets the Lafora disease phosphatase to glycogen. J Biol Chem 277:2377–2380. doi:10.1074/jbc.C100686200
Worby CA, Gentry MS, Dixon JE (2006) Laforin, a dual specificity phosphatase that dephosphorylates complex carbohydrates. J Biol Chem 281:30412–30418. doi:10.1074/jbc.M606117200
Rao SN, Maity R, Sharma J, Dey P, Shankar SK, Satishchandra P, Jana NR (2010) Sequestration of chaperones and proteasome into Lafora bodies and proteasomal dysfunction induced by Lafora disease-associated mutations of malin. Hum Mol Genet 19:4726–4734. doi:10.1093/hmg/ddq407
Garyali P, Siwach P, Singh PK, Puri R, Mittal S, Sengupta S, Parihar R, Ganesh S (2009) The malin–laforin complex suppresses the cellular toxicity of misfolded proteins by promoting their degradation through the ubiquitin–proteasome system. Hum Mol Genet 18:688–700. doi:10.1093/hmg/ddn398
Liu Y, Wang Y, Wu C, Zheng P (2009) Deletions and missense mutations of EPM2A exacerbate unfolded protein response and apoptosis of neuronal cells induced by endoplasm reticulum stress. Hum Mol Genet 18:2622–2631. doi:10.1093/hmg/ddp196
Vernia S, Rubio T, Heredia M, Rodríguez de Córdoba S, Sanz P (2009) Increased endoplasmic reticulum stress and decreased proteasomal function in Lafora disease models lacking the phosphatase laforin. PLoS ONE 4:e5907. doi:10.1371/journal.pone.0005907
Zeng L, Wang Y, Baba O, Zheng P, Liu Y (2012) Laforin is required for the functional activation of malin in endoplasmic reticulum stress resistance in neuronal cells. FEBS J 279:2467–2478. doi:10.1111/j.1742-4658.2012.08627.x
Rao SN, Sharma J, Maity R, Jana NR (2010) Co-chaperone CHIP stabilizes aggregate-prone malin, a ubiquitin ligase mutated in Lafora disease. J Biol Chem 285:1404–1413. doi:10.1074/jbc.M109.006312
Criado O, Aguado C, Gayarre J, Duran-Trio L, Garcia-Cabrero AM, Vernia S, San Millan B, Heredia M, Romá-Mateo C, Mouron S, Juana-Lopez L, Dominguez M, Navarro C, Serratosa JM, Sanchez M, Sanz P, Bovolenta P, Knecht E, Rodriguez de Cordoba S (2012) Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum Mol Genet 21:1521–1533. doi:10.1093/hmg/ddr590
Aguado C, Sarkar S, Korolchuk VI, Criado O, Vernia S, Boya P, Sanz P, Rodríguez de Córdoba S, Knecht E, Rubinsztein DC (2010) Laforin, the most common protein mutated in Lafora disease, regulates autophagy. Hum Mol Genet 19:2867–2876. doi:10.1093/hmg/ddq190
García-Giménez JL, Seco-Cervera M, Aguado C, Romá-Mateo C, Dasi F, Priego S, Markovic J, Knecht E, Sanz P, Pallardo FV (2013) Lafora disease fibroblasts exemplify the molecular interdependence between thioredoxin 1 and the proteasome in mammalian cells. Free Radic Biol Med 65:347–359. doi:10.1016/j.freeradbiomed.2013.07.001
Dutta D, Xu J, Kim JS, Dunn WA Jr, Leeuwenburgh C (2013) Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy 9:328–344. doi:10.4161/auto.22971
Kiffin R, Bandyopadhyay U, Cuervo AM (2006) Oxidative stress and autophagy. Antioxid Redox Signal 8:152–162. doi:10.1089/ars.2006.8.152
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441:523–540. doi:10.1042/BJ20111451
Navarro-Yepes J, Burns M, Anandhan A, Khalimonchuk O, Del Razo LM, Quintanilla-Vega B, Pappa A, Panayiotidis MI, Franco R (2014) Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid Redox Signal. doi:10.1089/ars.2014.5837
Aguiar CC, Almeida AB, Araujo PV, de Abreu RN, Chaves EM, do Vale OC, Macedo DS, Woods DJ, Fonteles MM, Vasconcelos SM (2012) Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev 2012:795259. doi:10.1155/2012/795259
Garcia-Gimenez JL, Gimeno A, Gonzalez-Cabo P, Dasi F, Bolinches-Amoros A, Molla B, Palau F, Pallardo FV (2011) Differential expression of PGC-1alpha and metabolic sensors suggest age-dependent induction of mitochondrial biogenesis in Friedreich ataxia fibroblasts. PLoS ONE 6:e20666. doi:10.1371/journal.pone.0020666
Viña J, Lloret A, Valles SL, Borras C, Badia MC, Pallardo FV, Sastre J, Alonso MD (2007) Mitochondrial oxidant signalling in Alzheimer’s disease. J Alzheimers Dis 11:175–181
Esteve JM, Armengod ME, Knecht E (2010) BRCA1 negatively regulates formation of autophagic vacuoles in MCF-7 breast cancer cells. Exp Cell Res 316:2618–2629. doi:10.1016/j.yexcr.2010.06.019
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Ganesh S, Delgado-Escueta AV, Sakamoto T, Avila MR, Machado-Salas J, Hoshii Y, Akagi T, Gomi H, Suzuki T, Amano K, Agarwala KL, Hasegawa Y, Bai DS, Ishihara T, Hashikawa T, Itohara S, Cornford EM, Niki H, Yamakawa K (2002) Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum Mol Genet 11:1251–1262. doi:10.1093/hmg/11.11.1263
Colinge J, Masselot A, Giron M, Dessingy T, Magnin J (2003) OLAV: towards high-throughput tandem mass spectrometry data identification. Proteomics 3:1454–1463. doi:10.1002/pmic.200300485
Fransson A, Ruusala A, Aspenstrom P (2003) Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem 278:6495–6502. doi:10.1074/jbc.M208609200
Kato T, Kapczinski F, Berk M (2010) Mitochondrial dysfunction and oxidative stress. In: Bipolar disorder. Wiley, p 244–254. doi:10.1002/9780470661277.ch18
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. doi:10.1038/nature05292
Du G, Mouithys-Mickalad A, Sluse FE (1998) Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free Radical Biol Med 25:1066–1074. doi:10.1016/S0891-5849(98)00148-8
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Liu XY, Yang JL, Chen LJ, Zhang Y, Yang ML, Wu YY, Li FQ, Tang MH, Liang SF, Wei YQ (2008) Comparative proteomics and correlated signaling network of rat hippocampus in the pilocarpine model of temporal lobe epilepsy. Proteomics 8:582–603. doi:10.1002/pmic.200700514
Jiang W, Du B, Chi Z, Ma L, Wang S, Zhang X, Wu W, Wang X, Xu G, Guo C (2007) Preliminary explorations of the role of mitochondrial proteins in refractory epilepsy: some findings from comparative proteomics. J Neurosci Res 85:3160–3170. doi:10.1002/jnr.21384
Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301:513–517. doi:10.1126/science.1083995
Fisher AB (2011) Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A(2) activities. Antioxid Redox Signal 15:831–844. doi:10.1089/ars.2010.3412
Baraibar MA, Hyzewicz J, Rogowska-Wrzesinska A, Ladouce R, Roepstorff P, Mouly V, Friguet B (2011) Oxidative stress-induced proteome alterations target different cellular pathways in human myoblasts. Free Radic Biol Med 51:1522–1532. doi:10.1016/j.freeradbiomed.2011.06.032
Chevallet M, Wagner E, Luche S, van Dorsselaer A, Leize-Wagner E, Rabilloud T (2003) Regeneration of peroxiredoxins during recovery after oxidative stress: only some overoxidized peroxiredoxins can be reduced during recovery after oxidative stress. J Biol Chem 278:37146–37153. doi:10.1074/jbc.M305161200
Jeong J, Kim Y, Kyung Seong J, Lee KJ (2012) Comprehensive identification of novel post-translational modifications in cellular peroxiredoxin 6. Proteomics 12:1452–1462. doi:10.1002/pmic.201100558
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4:600–609. doi:10.1038/ncpneuro0924
Lehtinen MK, Tegelberg S, Schipper H, Su H, Zukor H, Manninen O, Kopra O, Joensuu T, Hakala P, Bonni A, Lehesjoki AE (2009) Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J Neurosci 29:5910–5915. doi:10.1523/JNEUROSCI.0682-09.2009
Chang SJ, Yu BC (2010) Mitochondrial matters of the brain: mitochondrial dysfunction and oxidative status in epilepsy. J Bioenerg Biomembr 42:457–459. doi:10.1007/s10863-010-9317-4
Singh S, Sethi I, Francheschetti S, Riggio C, Avanzini G, Yamakawa K, Delgado-Escueta AV, Ganesh S (2006) Novel NHLRC1 mutations and genotype-phenotype correlations in patients with Lafora’s progressive myoclonic epilepsy. J Med Genet 43:e48. doi:10.1136/jmg.2005.039479
Patel M (2004) Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med 37:1951–1962. doi:10.1016/j.freeradbiomed.2004.08.021
Kudin AP, Zsurka G, Elger CE, Kunz WS (2009) Mitochondrial involvement in temporal lobe epilepsy. Exp Neurol 218:326–332. doi:10.1016/j.expneurol.2009.02.014
Liang LP, Ho YS, Patel M (2000) Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience 101:563–570
Yata K, Oikawa S, Sasaki R, Shindo A, Yang R, Murata M, Kanamaru K, Tomimoto H (2011) Astrocytic neuroprotection through induction of cytoprotective molecules; a proteomic analysis of mutant P301S tau-transgenic mouse. Brain Res 1410:12–23. doi:10.1016/j.brainres.2011.06.064
Yun HM, Jin P, Han JY, Lee MS, Han SB, Oh KW, Hong SH, Jung EY, Hong JT (2013) Acceleration of the development of Alzheimer’s disease in amyloid beta-infused peroxiredoxin 6 overexpression transgenic mice. Mol Neurobiol 48:941–951. doi:10.1007/s12035-013-8479-6
Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T (2009) Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging 1:425–437
Acknowledgments
This work was supported by grants from the Spanish Ministry of Education and Science (SAF2011-27442, BFU2011-22630), Fundació La Marató de TV3 (ref. 100130), EU-Funded FRAILOMIC-HEALTH.2012.2.1.1, TREAT-CMT IRDiRC consortium Research fellowship, Generalitat Valenciana (Prometeo 2009/051, Prometeo 2012/061) and an ACCI2012 action from CIBERER, an initiative of the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carlos Romá-Mateo Carmen Aguado and José Luis García-Giménez contributed equally to this work.
Rights and permissions
About this article
Cite this article
Romá-Mateo, C., Aguado, C., García-Giménez, J.L. et al. Increased Oxidative Stress and Impaired Antioxidant Response in Lafora Disease. Mol Neurobiol 51, 932–946 (2015). https://doi.org/10.1007/s12035-014-8747-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8747-0