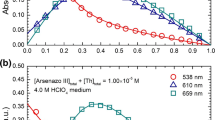
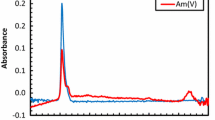
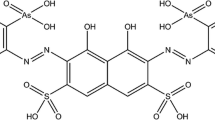
Abstract
Highly concentrated alkaline gallate solutions with 0.23≤[Ga(III)]T≤2.32 mol⋅dm−3 and 1≤[NaOH]T≤15 mol⋅dm−3 have been prepared and investigated by Raman and 71Ga-NMR spectroscopy. Both the Raman and 71Ga-NMR spectra are consistent with the presence of only one Ga-bearing species in these solutions, the tetrahedral hydroxocomplex, Ga(OH) −4 . Contact ion pairs were found to cause variations in the Raman and 71Ga-NMR parameters that are at the edge of detectability. Other species that have been claimed to exist in the literature, like higher hydroxo complexes (i.e., Ga(OH) 3−6 ) or the μ-oxo-bridged dimer (i.e., (OH)3Ga-O-(OH) 2−3 ), were not detected by these spectroscopic techniques. If such solution species exist at all, their concentrations are below the detection limit of Raman and 71Ga-NMR spectroscopy. The behavior of gallium appears to be very similar to that of aluminium under identical conditions, except that the dimeric species detected in aluminate solutions is undetectable in analogous gallates.
Similar content being viewed by others
References
Pokrovski, G.S., Schott, J., Hazemann, J.-L., Farges, F., Pokrovsky, O.S.: An X-ray absorption fine structure and nuclear magnetic resonance spectroscopy study of gallium-silica complexes in aqueous solutions. Geochim. Cosmochim. Acta 66, 4203–4222 (2002)
Baes, C.F. Jr., Mesmer, R.E.: The Hydrolysis of Cations. Wiley, London (1976)
Diakonov, I., Pokrovski, G.S., Benezeth, P., Schott, J., Dandurand, J.-L., Escalier, J.: Gallium speciation in aqueous solution. Experimental study and modelling Part 1. Thermodynamic properties of Ga(OH) −4 to 300 °C. Geochim. Cosmochim. Acta 61, 1333–1343 (1997)
Benezeth, P., Diakonov, I., Pokrovski, G.S., Dandurand, J.-L., Schott, J., Khodakovsky, I.L.: Gallium speciation in aqueous solution. Experimental study and modelling Part 2. Solubility of α-GaOOH in acidic solutions from 150 °C to 300 °C and hydrolysis constants of Ga(III) to 300 °C. Geochim. Cosmochim. Acta 61, 1345–1357 (1997)
Selvi, P., Ramasami, M., Samuel, M.H.P., Sripriya, R., Senhilkumar, K., Adaikkalam, P., Srinivasan, G.N.: Gallium recovery from Bayer liquor using hydroxamic acid resin. J. Appl. Polym. Sci. 92, 847–855 (2004)
Akitt, J.W.: Multinuclear studies of aluminium compounds. Prog. NMR Spectrosc. 24, 1–149 (1989)
Bradley, S.M., Kydd, R.A., Yamdagni, R.: Comparison of the hydrolysis of Ga(III) and Al(III) solutions by nuclear magnetic resonance spectroscopy. J. Chem. Soc., Dalton Trans. 2653–2656 (1990)
Akitt, J.W., Kettle, D.: 71Ga-NMR investigation of aqueous Ga(III) and its hydrolysis. Magn. Reson. Chem. 27, 377–379 (1989)
Biryuk, E.A., Nazarenko, V.A.: Determination of the hydrolysis constant of monomeric gallium(III) ions in solution with 0.1–1.0 M ionic strength. Zh. Neorg. Khim. 18, 2964–2967 (1973)
Akitt, J.W., Elders, J.M.: Multinuclear magnetic resonance studies of the hydrolysis of aluminium 8. Base hydrolysis monitored at very high magnetic field. J. Chem. Soc., Dalton Trans. 347–1355 (1988)
Öhman, L.-O., Edlund, U.: In: Grant, D.M., Harris, R.K. (eds.) Encyclopedia of NMR, p. 742. Wiley, London (1996)
Bradley, S.M., Kydd, R.A., Yamdagni, R.: Detection of a new polymeric species formed through the hydrolysis of Ga(III)-salt solutions. J. Chem. Soc., Dalton Trans. 413–417 (1990)
Parker, W.O. Jr., Millini, R., Kiricsi, I.: Metal substitution in Keggin-type tridecameric aluminium-oxo-hydroxy clusters. Inorg. Chem. 36, 571–575 (1997)
Chretien, A., Bizot, D.: Existence of the Ga(OH) −4 ion. Ser. C.: Sci. Chim. 266, 1688–1690 (1968)
Yatsenko, S.: Equilibrium constants for the reaction of Ga(OH)3 with NaOH solution. Akad. Nauk SSSR, Ural. Fil. 20, 153–156 (1970)
Sipos, P., May, P.M., Hefter, G.: Quantitative determination of an aluminate dimer in concentrated alkaline aluminate solutions by Raman spectroscopy. Dalton Trans. 368–375 (2006)
Moolenaar, R.J., Evans, J.C., McKeever, L.D.: The structure of the aluminate ion in solutions at high pH. J. Phys. Chem. 74, 3629–3636 (1970)
Gale, J.D., Rohl, A.L., Watling, H.R., Parkinson, G.M.: Theoretical investigation of the nature of aluminium containing species present in alkaline solution. J. Phys. Chem. B 102, 10372–10382 (1998)
Johansson, G.: The structure of the potassium aluminate K2[Al2O(OH)6]. Acta Chem. Scand. 20, 505–515 (1966)
Ivanov-Jemin, B.N., Zaitsev, B.E., Kaziev, G.Z., Gerasimova, T.Yu.: Alkali metzal gallates (MGaO2). Zh. Neorg. Khim. 24, 3230–3233 (1979)
Ivanov-Jemin, B.N., Kaziev, G.Z., Ivlieva, V.I., Aksenova, T.B.: X-ray diffraction study of cesium hydroxyaluminate- and hydroxygallate. Zh. Neorg. Chim. 26, 544–555 (1981)
Ivanov-Jemin, B.N., Kaziev, G.Z., Zaitsev, B.E., Nikolaeva, O.A.: Hydroxyaluminates and hydroxygallates of heavy alkaline metals. Koord. Khim. 7, 218–221 (1981)
Romanov, G.A., Kopylova, E.A., Zazubin, A., Nikolskaya, M.P.: IR-spectroscopic study of the properties and structure of gallate solutions. Trud. Inst. Matall. Obog., Akad. Nauk Kazakh. SSR 50, 13–22 (1975)
Brintzinger, H., Wallach, J.: About the hydroxo compounds. Angew. Chem. 47, 61–63 (1934)
Ljubimova, L.A., Ruzinov, L.P., Selokhova, N.P., Fomina, N.A.: Conductometric studies of gallium containing alkaline solutions. Elektrokhimiya 3, 1045–1047 (1967)
Sheka, I.A., Chaus, I.S., Mityureva, T.T.: The Chemistry of Gallium, p. 46. Elsevier, London (1966)
Gessner, W., Weinberger, M., Muller, D., Ni, L.P., Chaljapina, O.B.: On the crystalline phases of the systems K2O-Al2O3-H2O and Na2O-Al2O3-H2O. Z. Anorg. Allg. Chem. 547, 27–44 (1987)
Zabel, V., Schneider, M., Weinberger, M., Gessner, W.: Nonasodium-bis(hexahydroxoaluminate) trihydroxide hexahydrate. Acta Cryst. C 52, 747–749 (1996)
Weinberger, M., Schneider, M., Zabel, V., Muller, D., Gessner, W.: Nonasodium-bis (hexahydroxoaluminate) trihydroxide hexahydrate Na9[Al(OH)6]2(OH)3⋅6H2O—crystal structure. NMR spectroscopy and thermal behaviour. Z. Anorg. Allg. Chem. 622, 1799–1805 (1996)
Ivanov-Emin, B.N., Kaziev, G.Z., Ivlieva, V.I., Gerasimova, T.Yu.: X-ray diffraction studies of Ca- and Sr-hexahydroxo-aluminates and gallates. Zh. Fiz. Khim. 55, 255–257 (1981)
Ivanov-Emin, B.N., Rabovik, Ya.I.: Chemistry of gallium II. Hydroxy gallates of alkali and alkali earth metals. Zh. Obs. Khim. 17, 1061–1069 (1974)
Loeper, M., Gessner, W., Muller, D., Schneider, M.: About crystalline sodium hydroxyl-gallates. Z. Anorg. Allg. Chem. 623, 1483–1488 (1997)
Loeper, M., Schneider, M., Gessner, W., Reck, G.: Crystal structure of nonasodium-bis (hexahydroxoaluminate) trihydroxide hexahydrate Na9[Al(OH)6]2(OH)3⋅6H2O. Z. Krist. 211, 709–710 (1996)
Ivanov-Emin, B.N., Kaziev, G.Z., Gerasimova, T.Yu.: Study of the interaction of Ga(OH)3 and Ca(OH)2 in water at 25 °C. Izv. Vyss. Uch. Zaved., Khim. Khim. Techn. 25, 915–917 (1982)
Greenwood, N.N., Earnshow, A.: Chemistry of the Elements, p. 253. Pergamon Press, Elmsford (1984)
Cotton, F.A., Wilkinson, G.: Comprehensive Inorganic Chemistry, p. 217. Wiley, New York (1988)
Akitt, J.W., Gessner, W.J.: Aluminium-27 nuclear magnetic resonance investigation of highly alkaline aluminate solutions. J. Chem. Soc., Dalton Trans. 147–148 (1984)
Sipos, P., Hefter, G., May, P.M.: 27Al-NMR and Raman spectroscopic studies of alkaline aluminate solutions with extremely high caustic content—does the octahedral species Al(OH) 3−6 exist in solution? Talanta 70, 761–765 (2006)
Watling, H.R., Fleming, S.D., van Bronswijk, W., Rohl, A.: Ionic structure in caustic aluminate solutions and the precipitation of gibbsite. J. Chem. Soc., Dalton Trans. 3911–3917 (1998)
Sheka, I.A., Chaus, I.S., Mityureva, T.T.: The Chemistry of Gallium, p. 77. Elsevier, London (1966)
Sipos, P., May, P.M., Hefter, G.T.: Carbonate removal from concentrated hydroxide solutions. The Analyst 125, 955–958 (2000)
Sipos, P., Hefter, G.T., May, P.M.: Viscosities and densities of highly concentrated aqueous MOH solutions (M+ = Na+, Li+, K+, Cs+ and (CH3)4N+). J. Chem. Eng. Data 45, 613–617 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sipos, P., Megyes, T. & Berkesi, O. The Structure of Gallium in Strongly Alkaline, Highly Concentrated Gallate Solutions—a Raman and 71Ga-NMR Spectroscopic Study. J Solution Chem 37, 1411–1418 (2008). https://doi.org/10.1007/s10953-008-9314-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9314-y