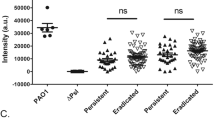
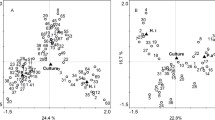
Abstract
The differentiation of bacterial biofilms in the airway environment, the pathogenesis of airway biofilm, and possible therapeutic methods are discussed. Biofilm diseases that characteristically involve the respiratory system include cystic fibrosis (CF), diffuse panbronchiolitis (DPB), and bronchiectasia with Pseudomonas aeruginosa (P. aeruginosa) infection. There is evidence to suggest that almost all strains of P. aeruginosa have the genetic capacity to synthesize alginate, a main matrix of biofilms, when ecological conditions are unfavorable for their survival. The bacteria inside the mature biofilm show increased resistance to both antibacterials and phagocytic cells, express fewer virulence factors because of their stationary state of growth, and are less stimulatory to the mucosa because of the ‘sandwich binding’. These factors facilitate both the colonization of bacteria and their extended survival even under unfavorable conditions. Since the biofilm limits colonization to a latent form, the clinical symptoms in this situation are unremarkable. However, the clinical progression of both CF and DPB proceeds in two characteristic directions. The first is an acute exacerbation caused by planktonic bacteria that have germinated from the biofilm. The second is a slow progression of disease that is induced by harmful immune reactions. The harmful reactions are mediated by alginate, which induces antigen antibody reactions around the airways, as well as formation of circulating immune complexes that are deposited on lung tissue. Furthermore, the highest titer of bacterial permeability increasing anti-neutrophil cytoplasmic autoantibodies (BPI-ANCA) is observed in association with highly impaired pulmonary function in patients with CF and DPB, as well as in patients with a lengthy period of colonization with P. aeruginosa. BPI-ANCA subsequently makes chronic airway infection even more intractable. The long-term use of 14- or 15-ring membered macrolides results in a favorable clinical outcome for patients with DPB and in some patients with CF. In the last 10 years, an increasing number of studies have reported secondary actions of macrolides that include effects on both airway and phagocytic cells, as well as an anti-biofilm activity. The 14- or 15-ring membered macrolides inhibit: (i) the alginate production from P. aeruginosa; (ii) the antibody reaction to alginate, which leads to a decrease in the immune complex formation; and (iii) the activation of the autoinducer 3-O-C12-homoserine lactone and subsequent expression of lasI and rhlI in quorum sensing systems in P. aeruginosa. These anti-biofilm actions of macrolides may represent their basic mechanisms of action on airway biofilm disease.










Similar content being viewed by others
References
Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sei Am 1978; 238: 86–95
Costerton JW, Irvin RT. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol 1981; 35: 299–324
Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother 2001; 45: 999–1007
Koch C, Høiby N. Pathogenesis of cystic fibrosis. Lancet 1993; 341: 1065–9
Kobayashi H. Airway biofilni disease: its clinical manifestation and therapeutic possibilities of macrolides. J Infect Chemother 1995; 1: 1–15
Linker A, Jones RS. A new polysaccharide resembling alginic acid isolated from pseudomonas. J Biol Chem 1966; 241: 3845–51
Martin DR. Mucoid variation in Pseudomonas aeruginosa induced by the action of phage. J Med Microbiol 1973; 6: 111–8
Fyfe JA, Govan JR. Alginate synthesis in mucoid Pseudomonas aeruginosa: a chromosomal locus involved control. J Gen Microbiol 1980; 119 (Pt 2): 443–50
Miller RV, Rubero VJR. Mucoid conversion by phages of Pseudomonas aeruginosa strains from patients with cystic fibrosis. J Clin Microbiol 1984; 19: 717–9
Terry JM, Pina SE, Mattingly SJ. Environmental conditions which influence mucoid conversion in Pseudomonas aeruginosa PAO 1. Infect Immun 1991; 59: 471–7
Govan JR, Fyfe JA. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother 1978; 4: 233–40
Knowles MR, Stutts MJ, Spock A, et al. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science 1983; 221: 1067–70
Berry A, DeVault JD, Chakrabarty AM. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol 1989; 171: 2312–7
Davies DG, Chakrabarty AM, Geesey GG. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl Environ Microbiol 1993; 59: 1181–6
Piggott NH, Sutherland IW, Jarman TR. Alginate synthesis by mucoid strains of Pseudomonas aeruginosa PAO. Eur J Appl Microbiol Biotechnol 1982; 16: 131–5
Gambello MJ, Iglewski BH. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol 1991; 173: 3000–9
Zielinski NA, Maharaj R, Roychoudhury S, et al. Alginate synthesis in Pseudomonas aeruginosa: environmental regulation of the algC promoter. J Bacteriol 1992; 174: 7680–8
Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev 2002; 54: 1359–71
Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 2002; 109: 317–25
Martin DW, Schurr MJ, Mudd MH, et al. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci U S A 1993; 90: 8377–81
Mathee K, McPherson CJ, Ohman DE. Posttrans lational control of the algT(algU)-encoded sigma22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins Muc A and MucB (AlgN). J Bacteriol 1997; 179: 3711–20
Schurr MJ, Yu H, Martinez-Salazar JM, et al. Control of AlgU, a member of the sigma E-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J Bacteriol 1996; 178: 4997–5004
Malhotra S, Silo-Suh LA, Mathee K, et al. Proteome analysis of the effect of mucoid conversion on global protein expression in Pseudomonas aeruginosa strain PAO1 shows induction of the disulfide bond isomerase, dsbA. J Bacteriol 2000; 182: 6999–7006
Mathee K, Ciofu O, Sternberg C, et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 1999; 145: 1349–57
Davies DG, Parsek MR, Pearson JP, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998; 280: 295–8
Passador L, Cook JM, Gambello MJ, et al. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 1993; 260: 1127–30
Pearson JP, Gray KM, Passador L, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A 1994; 91: 197–201
Ochsner UA, Koch AK, Fiechter A, et al. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 1994; 176: 2044–54
McLean RJC, Whiteley M, Stickler DJ, et al. Evidence of autoinducer activity in naturally occurring biofilm. FEMS Microbiol Lett 1997; 154: 259–63
Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol 1995; 177: 7155–63
Winson MK, Camara M, Latifi A, et al. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginsoa. Proc Natl Acad Sci U S A 1995; 92: 9427–31
Gambello MJ, Kaye S, Iglewski BH. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun 1993; 61: 1180–4
Toder DS, Gambello MJ, Iglewski BH. Pseudomonas aeruginosa LasA: a second elastase gene under transcriptional control of lasR. Mol Microbiol 1991; 5: 2003–10
Pearson JP, Passador L, Iglewski BH, et al. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 1995; 92: 1490–4
Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun 2000; 68: 4839–49
Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol 2003; 6: 56–60
Singh PK, Schaefer AL, Parsek MR, et al. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000; 407: 762–4
Charlton TS, de Nys R, Netting A, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2000; 2: 530–41
Erickson DL, Endersby R, Kirkham A, et al. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect Immun 2002; 70: 1783–90
Wu H, Song Z, Hentzer M, et al. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology 2000; 146: 2481–93
DiMango E, Zar HJ, Bryan R, et al. Diverse Pseudomonas aeruginosa gene products stimulate respirator epithelial cells to produce interleukin-8. J Clin Invest 1995; 96: 2204–10
Smith RS, Fedyk ER, Springer TA, et al. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J Immunol 2001; 167: 366–74
Telford G, Wheeler D, Williams P, et al. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone has immunomodulatory activity. Infect Immun 1998; 66: 36–42
Smith RS, Harris SG, Phipps R, et al. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol 2002; 184: 1132–9
O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 1998; 30: 295–304
Ramphal R, Vishwanath S. Why is Pseudomonas the colonizer and why does it persist? Infection 1987; 15: 281–7
Houdret NR, Ramphal A, Scharfman JM, et al. Evidence for the in vivo degradation of human respiratory mucins during Pseudomonas aeruginosa infection. Biochim Biophys Acta 1989; 992: 96–105
Saiman L, Ishimoto K, Lory S, et al. The effect of pilliation and exoproduct expression on the adherence of Pseudomonas aeruginosa to respiratory epithelial monolayers. J Infect Dis 1990; 161: 541–8
Ramphal R. The adhesion-receptor system of Pseudomonas aeruginosa: where are we now? Pediatr Pulmonol 1991; 6 Suppl. 9.14: 140–1
Prince A. Mini-review-adhesions and receptors of Pseudomonas aeruginosa associated with infection of the respiratory tract. Microb Pathog 1992; 13: 251–60
Ghigo JM. Natural conjugative plasmids induce bacterial biofilm development. Nature 2001; 412: 442–5
Pearson JP, Pesci EC, Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 1997; 179: 5756–67
Kievit TR, Gillis R, Marx S, et al. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl Environ Microbiol 2001; 67: 1865–73
Latifi A, Foglino M, Tanaka K, et al. A hierarchical quorum-sensing cascade in Pseudonomas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol 1996; 21: 1137–46
Pesci EC, Pearson JP, Seed PC, et al. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 1997; 179: 3127–32
Boyd A, Chakrabarty AM. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol 1994; 60: 2355–9
Gacesa P. Alginate-modifying-enzymes: a proposed unified mechanism of action for the lyases and epimerases. FEBS Lett 1987; 212: 199–202
Ohgaki N. Bacterial biofilm in chronic airway infection. Kansenshogaku Zasshi 1994; 68: 138–51
Luzar MA, Montie TC. A virulence and altered physiological properties of cystic fibrosis strains of Pseudomonas aeruginosa. Infect Immun 1985; 50: 572–6
Woods DE, Sokal DA, Bryan LF, et al. In vitro regulation of virulence in Pseudomonas aeruginosa associated with genetic rearrangement. J Infect Dis 1991; 163: 143–9
Fagan M, Francis P, Hayward AC, et al. Phenotypic conversion of Pseudomonas aeruginosa in cystic fibrosis. J Clin Microbiol 1990; 28: 1143–6
Oliver AM, Weir DM. Inhibition of bacterial binding to mouse macrophages by pseudomonas alginate. J Clin Lab Immunol 1983; 10: 221–4
Bayer AS, Speert DP, Park S, et al. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of Pseudomonas aeruginosa. Infect Immun 1991; 59: 302–8
Jensen ET, Kharazmi A, Lam K, et al. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect Immun 1990; 58: 2383–5
Schwarzmann S, Boring JR. Antiphagocytic effect of slime from a mucoid strain of Pseudomonas aeruginosa. Infect Immun 1971; 3: 762–7
Kobayashi H, Ohgaki N, Takeda H. Therapeutic possibilities for diffuse panbronchiolitis. Int J Antimicrob Agent 1993; 3: 81–6
Ishida H, Ishida Y, Kurosaka Y, et al. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agents Chemother 1998; 42: 1641–5
Stewart PS. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng 1998; 59: 261–72
Stewart PS. Diffusion in biofilms. J Bacteriol 2003; 85: 1485–91
Brown MR, Allison DG, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother 1988; 22: 777–80
Xu KD, Stewart PS, Xia F, et al. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol 1998; 64: 4035–9
Zahler J, Stewart PS. Transmission electron microscopic study of antibiotic action on Klebsiella pneumoniae biofilm. Antimicrob Agents Chemother 2002; 46: 2679–83
Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993; 73: 1251–4
Homma H, Yamanaka A, Tanimoto S, et al. Diffuse panbronchitis: a disease of the transitional zone of the lung. Chest 1983; 83: 63–9
Reynolds HY, di Sant’ Agnese PA, Zierdt CH. Mucoid Pseudomonas aeruginosa: a sign of cystic fibrosis in young adults with chronic pulmonary disease? JAMA 1976; 236: 2190–2
Fick Jr RB. Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest 1989; 96: 158–64
Høiby N, Koch C. Cystic fibrosis: Pseudomonas aeruginosa infection in cystic fibrosis and its management. Thorax 1990; 45: 881–4
Pier GB. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current statues of the host-bacterium infection. J Infect Dis 1985; 151: 575–80
Høiby N. Pseudomonas aeruginosa infection in cystic fibrosis: relation between mucoid strain of Pseudomonas aeruginosa. Acta Pathol Microbiol Scand [B] Microbiol Immunol 1974; 82: 551–8
Brown ML, Aldrich HC, Gauthier JJ. Relationship between glycocalyx and povidone-iodine resistance in Pseudomonas aeruginosa (ATCC 27853) biofilms. Appl Environ Microbiol 1995; 61: 187–93
Kobayashi H. Clinical use of levofloxacin for intractable RTI in outpatients. Penetration 1998 (annual issue); 31–5
Bryan LE, Kureiski A, Rabin HR. Detection of antibodies to Pseudomonas aeruginosa alginate extracellular polysaccharide in animals and cystic fibrosis patients by enzyme-linked immunosorbent assay. J Clin Microbiol 1983; 18: 276–82
Woods DE, Bryan LE. Studies on the ability of alginate to act as a protective immunogen against infection with Pseudomonas aeruginosa in animals. J Infect Dis 1985; 151: 581–8
Fick RB. Pathogenetic mechanisms in cystic fibrosis lung disease: a paradigm for inflammatory airways disease. J Lab Clin Med 1993; 121: 632–4
Høiby N, Döring G, Schiøtz PO. The role of immune complex in the pathogenesis of bacterial infections. Annu Rev Microbiol 1986; 40: 29–53
Høiby N, Giwercman B, Jensen ET, et al. Immune response in cystic fibrosis-helpful or harmful? In: Escobar H, Baguero CF, Suárez L, editors. Clinical ecology of cystic fibrosis. Elsevier Science Publishers, Amsterdam, 1993: 133–41
Hornick DB, Fick Jr RB. The immunoglobulin G subclass composition of immune complexes in cystic fibrosis: implications for the pathogenesis of the Pseudomonas lung lesion. J Clin Invest 1990; 86: 1285–92
Tomashefski J Jr, Abramowsky CR, Dahms BB. The pathology of cystic fibrosis. In: Davis PB, editor. Cystic fibrosis: lung biology in health and disease. New York (NY): Marcel Dekker Inc, 1993: 64, 435-89
Kobayashi H. Effective mechanism of macrolide antibiotics on diffuse panbronchiolitis [in Japanese]. Jpn J Chemother 1995; 43: 96–101
Kronborg G, Shand GH, Fomsgaard A, et al. Lipopolysaccharide is present in immune complexes isolated from sputum in patients with cystic fibrosis and chronic Pseudomonas aeruginosa lung infection. APMIS 1992; 100: 175–80
Weiss J, Victor M, Stendahl O, et al. Killing of gram-negative bacteria by polymorphonuclear leukocytes: role of an O2-independent bactericidal system. J Clin Invest 1982; 69: 959–70
Zhao MH, Johnes SJ, Lockwood CM. Bactericidal/permeability-increasing protein (BPI) is an important antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in vasculitis. Clin Exp Immunol 1995; 99: 49–56
Zhao MH, Jayne DRW, Ardies LG, et al. Autoantibodies against bactericidal/ permeability increasing protein in patients with cystic fibrosis. Q J Med 1998; 89: 259–65
Kobayashi O. Clinical role of autoantibody against bactericidal/permeability-increasing protein in chronic airway infection. J Infect Chemother 1998; 4: 83–93
Ohtami S, Kobayashi O, Ohtami H. Analysis of intractable factors in chronic airway infections: role of the autoimmunity induced by BPI-ANCA. J Infect Chemother 2001; 7: 228–38
Thomas FB. Cystic fibrosis. In: Murray JF, editor. Textbook of Respiratory Medicine. Philadelphia (PA): Saunders, 1988: 1126–52
Kudoh S, Uetake K, Hagiwara K, et al. A study on the clinical effect of low-dose, long-term administration of erythromycin on diffuse panbronchiolitis. The therapeutic results of 4 years [in Japanese]. Nihon Kyobu Shikkan Gakkai Zasshi 1987; 25: 632–42
Sawaki M, Mikami R, Mikasa K, et al. A study on long-term chemotherapy with erythromycin in patients with chronic lower respiratory infection [in Japanese]. Kansenshogaku Zasshi 1986; 60: 37–44
Yamamoto M, Kondo A, Tamura M, et al. Long-term therapeutic effects of erythromycin chemotherapy and new quinolone antibacterial agents on diffuse panbronchiolitis-retrospective comparative design [in Japanese]. Nihon Kyobu Shikkan Gakkai Zasshi 1990; 28: 1305–15
Yamamoto M. Annual report on the study of diffuse disseminated disease [in Japanese]. Grant-in-Aid from the Ministry of Health and Welfare in Japan. The therapeutic effect of erythromycin on DPB, a double blind comparative study. Report Book to Ministry of Health and Welfare Japan 1992, 18–20
Naess A, Solberg CO. Effects of two macrolide antibiotics on human leukocyte membrane receptors and functions. APMIS 1988; 96: 503–8
Takeda H, Miura H, Kobayashi H, et al. A study on long-term administration of TE-031 in patients with diffuse panbronchiolitis [in Japanese]. Kansenshogaku Zasshi 1989; 63: 71–8
Kobayashi H, Shimada K, Sano Y, et al. Study on azithromycin in treatment of diffuse panbronchiolitis [in Japanese]. Kansenshogaku Zasshi 1995; 69: 711–22
Jaffé A, Francis J, Rosental M, et al. Long-term azithromycin may improve lung function in children with cystic fibrosis [letter]. Lancet 1998; 351: 420
Equi A, Balfour-Lynn IM, Bush A, et al. Long term azithromycin in children with cystic fibrosis: a randomized, placebo-controlled crossover trial. Lancet 2002; 360: 978–84
Wolter J, Seeney S, Bell S, et al. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002; 57: 212–6
Tamaoki A, Sakai N, Isono K, et al. Erythromycin inhibition transport across airway epithelial cells in dogs [in Japanese]. Respiration 1990; 9: 1036–9
Miyake M, Taki F, Taniguchi H. Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma. Chest 1991; 99: 670–5
Ramphal R, Guay C, Pier GB. Pseudomonas aeruginosa adhesions for tracheobronchial mucin. Infect Immun 1987; 55: 600–3
Nakashio S, Susa C, Qiu S, et al. Antimicrobial activity of clarithromycin and its effect on bacterial adherence to medical material. Jpn J Antibiot 1993; 46: 428–36
Yamasaki T. Adherence of Pseudomonas aeruginosa to mouse tracheal epithelium: the effect of antimicrobial agents [in Japanese]. Kansenshogaku Zasshi 1990; 64: 575–83
Kobayashi H. Bacteria-host-interaction [in Japanese]. Respiration 1990; 9: 510–21
Baumann U, Fischerr JJ, Gudowius P, et al. Buccal adherence of Pseudomonas aeruginosa in patients with cystic fibrosis under long-term therapy with azithromycin. Infection 2001; 29: 7–11
Johanson JD. Antibiotic uptake by alveolar macrophage. J Lab Clin Med 1980; 95: 429–36
Tulkens PM. Intracellular activity. In: Neu HC, Young LS, Zinner SH, editors. The new macrolides, azalides and streptogramines. New York (NY): Marcel Dekker Inc, 1993: 41–6
Katahira J, Kikuchi K, Shibata Y, et al. Kinetic studies on the effect of macrolides on cytokine production [in Japanese]. Chemotherapy 1991; 39: 678–86
Lino Y, Toriyama M, Kudo K, et al. Erythromycin inhibition of lipopolysaccharide-stimulated tumor necrosis factor alpha production by human monocytes in vitro. Ann Otol Rhinol Laryngol Suppl 1992; 157: 16–20
Schultz MJ, Speelman P, Zaat S, et al. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob Agents Chemother 1998; 42: 1605–9
Kawasaki S, Takizawa H, Ohtoshi T, et al. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob Agents Chemother 1998; 42: 1499–502
Oda H, Kadota J, Kohno S, et al. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest 1994; 106: 1116–23
Kadota J, Sakito O, Kohno S, et al. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis 1993; 147: 153–9
Abe S, Nakamura H, Inoue S, et al. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2000; 22: 51–60
Desaki M, Takizawa H, Ohtoshi T, et al. Erythromycin suppresses nuclear factor-kappaB and activator protein-1 activation in human bronchial epithelial cells. Biochem Biophys Res Commun 2000; 267: 124–8
Arioka H. Effects of erythromycin on neutrophil chemotactic activity (conference paper) [in Japanese]. Therapeutic Research 1990, 77
Anderson R. Erythromycin and roxithromycin potentiate human neutrophil locomotion in vitro by inhibition of leukoattractant-activated Superoxide generation and autooxidation. J Infect Dis 1989; 159: 966–73
Villagrasa V, Berto L, Cortijo J, et al. Effects of erythromycin on chemoattractant-activated human polymorphonuclear leukocytes. Gen Pharmacol 1997; 29: 605–9
Hand WL, Hand DL, King-Thompson NL. Antibiotic inhibition of the respiratory burst response in human polymorphonuclear leukocytes. Antimicrob Agents Chemother 1990; 34: 863–70
Van Rensburg CEJ, Anderson R, Joone M, et al. Effects of erythromycin on cellular and humoral immune functions in vitro and in vivo. J Antimicrob Chemother 1981; 8: 467–74
Ohtami H. Study on the pathogenic role of alginate production by mucoid pseudomonas aeruginases in diffuse panbronchitis (in Japanese). Jap J Infect Dis 1996; 69: 553–67
Kobayashi H. Chronic bronchitis. In: Neu HC, Young LS, Zinner SH, editors. The new macrolides azalides and streptgramins. New York (NY): Marcel Dekker Inc, 1993: 125–9
Fujimaki K, Ikeda K, Takahata M, et al. Characteristics of biofilm formed by Pseudomonas aeruginosa in vitro [in Japanese]. Chemotherapy 1992; 40: 886–93
Nagino K, Kobayashi H. Influence of macrolides on mucoid alginate biosynthetic enzyme from Pseudomonas aeruginosa. Clin Microbiol Infect 1997; 3: 432–9
Kobayashi O, Moser C, Jensen PO, et al. Azithromycin treatment inhibits induction of mucoid phenotype in susceptible BALB/c mice with chronic Pseudomonas aeruginosa lung infection [abstract]. Proceedings of XIIIth International Cystic Fibrosis Congress; Stockholm, Sweden. Cystic Fibrosis Assoc. Lundin (Sweden): Inge-Britt, 2000: 164
Pederson SS, Kharazmi A, Espersen F. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect Immun 1990; 58: 3363–8
Roychoudhury S, May TB, Gill JF, et al. Purification and characterization of guanosine diphospho-D mannose dehydrogenase: a key enzyme in the biosynthesis of alginate by Pseudomonas aeruginosa. J Biol Chem 1989; 264: 9380–5
Roychoudhury S, Zielinski NA, De Vault JD, et al. Pseudomonas aeruginosa infection in cystic fibrosis: biosynthesis of alginate as a virulence factor. In: Homma JY, Tanimoto H, Holder IA, Høiby N, Döring G, editors. Pseudomonas eruginosa in human disease. Antibiot Chemother 1991; 44: 63–7
Pugashetti BK, Vadas L, Wood RE, et al. GDP-mannose dehydrogenase and biosynthesis of alginate like polysaccharide in a mucoid strain of Pseudomonas aeruginosa. J Bacteriol 1982; 153: 1107–10
Pearson JP, Feldman M, Iglewski BH, et al. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun 2000; 68: 4331–4
Van Delden C, Pesel EC, Pearson JP, et al. Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum sensing mutant. Infect Immun 1998; 66: 4499–502
Manefield M, de Nys R, Kumar N, et al. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone(AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999; 145: 283–91
Hentzer M, Riedel K, Rasmussen TB, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002; 148: 87–102
Hentzer M, Wu H, Anderson JB, et al. Attention of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 2003; 22: 3803–15
Tateda K, Comte R, Pechere JC, et al. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2001; 45: 1930–3
Engebrecht J, Nealson KH, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of the functions from Vibrio fischeri. Cell 1983; 32: 773–81
Acknowledgments
The author would like to thank Drs Hiroshi Ohtami, Hidehiro Watanabe, Hideaki Takeda and Osamu Kobayashi for collaboration of the biofilm research. Special thanks to Ms Sumiko Takahashi for assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, H. Airway Biofilms. Treat Respir Med 4, 241–253 (2005). https://doi.org/10.2165/00151829-200504040-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00151829-200504040-00003