Abstract
Objective
The purpose of this study was to develop an artificial intelligence (AI)–based model to detect features of atrial fibrillation (AF) on chest radiographs.
Methods
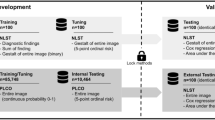
This retrospective study included consecutively collected chest radiographs of patients who had echocardiography at our institution from July 2016 to May 2019. Eligible radiographs had been acquired within 30 days of the echocardiography. These radiographs were labeled as AF–positive or AF–negative based on the associated electronic medical records; then, each patient was randomly divided into training, validation, and test datasets in an 8:1:1 ratio. A deep learning–based model to classify radiographs as with or without AF was trained on the training dataset, tuned with the validation dataset, and evaluated with the test dataset.
Results
The training dataset included 11,105 images (5637 patients; 3145 male, mean age ± standard deviation, 68 ± 14 years), the validation dataset included 1388 images (704 patients, 397 male, 67 ± 14 years), and the test dataset included 1375 images (706 patients, 395 male, 68 ± 15 years). Applying the model to the validation and test datasets gave a respective area under the curve of 0.81 (95% confidence interval, 0.78–0.85) and 0.80 (0.76–0.84), sensitivity of 0.76 (0.70–0.81) and 0.70 (0.64–0.76), specificity of 0.75 (0.72–0.77) and 0.74 (0.72–0.77), and accuracy of 0.75 (0.72–0.77) and 0.74 (0.71–0.76).
Conclusion
Our AI can identify AF on chest radiographs, which provides a new way for radiologists to infer AF.
Key Points
• A deep learning–based model was trained to detect atrial fibrillation in chest radiographs, showing that there are indicators of atrial fibrillation visible even on static images.
• The validation and test datasets each gave a solid performance with area under the curve, sensitivity, and specificity of 0.81, 0.76, and 0.75, respectively, for the validation dataset, and 0.80, 0.70, and 0.74, respectively, for the test dataset.
• The saliency maps highlighted anatomical areas consistent with those reported for atrial fibrillation on chest radiographs, such as the atria.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
Data generated or analyzed during the study are available from the corresponding author by request.
Abbreviations
- AF:
-
Atrial fibrillation
- AI:
-
Artificial intelligence
- AUC:
-
Area under the curve
- LVEF:
-
Left ventricular ejection fraction
- ROC:
-
Receiver operating characteristic curve
References
Commission CQ (2018) A national review of radiology reporting within the NHS in England. Care Quality Commission, London. https://www.cqc.org.uk/sites/default/files/20180718-radiology-reporting-review-report-final-for-web.pdf. Accessed 01 Apr 2021
Danzer CS (1919) The cardio-thoracic ratio: an index of cardiac enlargement. Am J Med Sci 157:513–518
Chugh SS, Havmoeller R, Narayanan K et al (2014) Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129(8):837–847
Lippi G, Sanchis-Gomar F, Cervellin G (2021) Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 16(2):217–221
Kannel WB, Wolf PA, Benjamin EJ, Levy D (1998) Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 82(8a):2n–9n
Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D (1998) Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98(10):946–952
Thrall G, Lane D, Carroll D, Lip GY (2006) Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 119(5):448.e441–419
Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ (2004) Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 90(3):286–292
Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL (2011) Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 4(3):313–320
Benjamin EJ, Muntner P, Alonso A et al (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139(10):e56–e528
Loy CT, Irwig L (2004) Accuracy of diagnostic tests read with and without clinical information: a systematic review. JAMA 292(13):1602–1609
Burgener F, Kormano M, Pudas T (2011) The chest X-ray (differential diagnosis in conventional radiology), 2nd edn. Thieme, Stuttgart New York
Mahesh PA, Vidyasagar B, Jayaraj BS (2007) Principles and interpretation of chest X-rays. Anshan Limited, Chennai India
Ueda D, Shimazaki A, Miki Y (2019) Technical and clinical overview of deep learning in radiology. Jpn J Radiol 37(1):15–33
Hinton G (2018) Deep learning—a technology with the potential to transform health care. JAMA 320(11):1101–1102
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521(7553):436–444
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1-39.e14
Tan M, Le Q (2019) Efficientnet: rethinking model scaling for convolutional neural networks. International Conference on Machine Learning, PMLR, pp 6105–6114
Paszke A, Gross S, Massa F et al (2019) Pytorch: an imperative style, high-performance deep learning library. arXiv preprint arXiv:191201703
Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D (2020) Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Comput Vis 128(2):336–359
Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA (1995) Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92(7):1954–1968
Abiyev RH, Ma'aitah MKS (2018) Deep convolutional neural networks for chest diseases detection. J Healthc Eng 2018:4168538
Arsalan M, Owais M, Mahmood T, Choi J, Park KR (2020) Artificial intelligence-based diagnosis of cardiac and related diseases. J Clin Med 9(3)
Cicero M, Bilbily A, Colak E et al (2017) Training and validating a deep convolutional neural network for computer-aided detection and classification of abnormalities on frontal chest radiographs. Invest Radiol 52(5):281–287
Que Q, Tang Z, Wang R et al (2018) CardioXNet: automated detection for cardiomegaly based on deep learning. Annu Int Conf IEEE Eng Med Biol Soc 2018:612–615
Rajpurkar P, Irvin J, Ball RL et al (2018) Deep learning for chest radiograph diagnosis: a retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med 15(11):e1002686
Toba S, Mitani Y, Yodoya N et al (2020) Prediction of pulmonary to systemic flow ratio in patients with congenital heart disease using deep learning-based analysis of chest radiographs. JAMA Cardiol 5(4):449–457
Zhou S, Zhang X, Zhang R (2019) Identifying cardiomegaly in ChestX-ray8 using transfer learning. Stud Health Technol Inform 264:482–486
Zou XL, Ren Y, Feng DY et al (2020) A promising approach for screening pulmonary hypertension based on frontal chest radiographs using deep learning: a retrospective study. PLoS One 15(7):e0236378
Dreyer KJ, Geis JR (2017) When machines think: radiology’s next frontier. Radiology 285(3):713–718
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Daiju Ueda.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• model development study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.31 mb)
Rights and permissions
About this article
Cite this article
Matsumoto, T., Ehara, S., Walston, S.L. et al. Artificial intelligence-based detection of atrial fibrillation from chest radiographs. Eur Radiol 32, 5890–5897 (2022). https://doi.org/10.1007/s00330-022-08752-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08752-0