Background
Clostridioides difficile (formerly Clostridium difficile) is a gram-positive, anaerobic, spore-forming bacillus that is responsible for the development of antibiotic-associated diarrhea and colitis. C difficile colitis results from a disturbance of the normal bacterial flora of the colon, colonization by C difficile, and the release of toxins that cause mucosal inflammation and damage.
Antibiotic therapy is a key factor that alters the colonic flora. C difficile infection (CDI) occurs primarily in hospitalized patients but also may be community-acquired. CDI commonly manifests as mild to moderate diarrhea, occasionally with abdominal cramping. Pseudomembranes (adherent, yellowish white plaques on the intestinal mucosa) are occasionally observed (see the images below). In rare cases, patients with CDI can present with an acute abdomen and fulminant, life-threatening colitis. (See Presentation.)
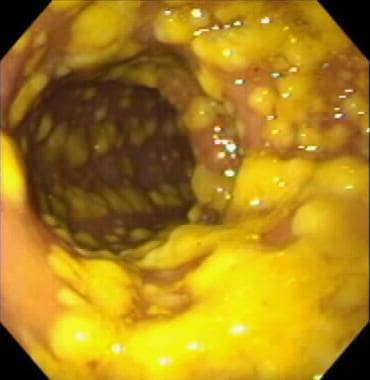 Clostridioides (Clostridium) Difficile Colitis. Endoscopic visualization of pseudomembranous colitis, a characteristic manifestation of full-blown C difficile colitis. Classic pseudomembranes are visible as raised, yellow plaques ranging from 2 to 10 mm in diameter and scattered over the colorectal mucosa. Courtesy of Gregory Ginsberg, MD, University of Pennsylvania.
Clostridioides (Clostridium) Difficile Colitis. Endoscopic visualization of pseudomembranous colitis, a characteristic manifestation of full-blown C difficile colitis. Classic pseudomembranes are visible as raised, yellow plaques ranging from 2 to 10 mm in diameter and scattered over the colorectal mucosa. Courtesy of Gregory Ginsberg, MD, University of Pennsylvania.
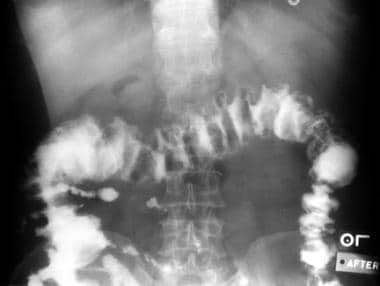 Clostridioides (Clostridium) Difficile Colitis. Barium enema demonstrating the typical serrated appearance of the barium column (resulting from trapped barium between the edematous mucosal folds and the plaquelike membranes of pseudomembranous colitis).
Clostridioides (Clostridium) Difficile Colitis. Barium enema demonstrating the typical serrated appearance of the barium column (resulting from trapped barium between the edematous mucosal folds and the plaquelike membranes of pseudomembranous colitis).
Approximately 20% of individuals who are hospitalized become colonized with C difficile during hospitalization, and more than 30% of these patients develop diarrhea. Thus, C difficile colitis is currently one of the most common nosocomial infections. (See Pathophysiology and Etiology.)
The diagnosis of C difficile colitis should be suspected in any patient with diarrhea who has received antibiotics within the previous 3 months, has been recently hospitalized, and/or has an occurrence of diarrhea 48 hours or more after hospitalization. [1] However, more recent studies have shown that C difficile can be the cause of diarrhea in community dwellers without previous hospitalization or antibiotic exposure [2] ; therefore, the diagnosis should be suspected in this population as well. (See Presentation and Workup.)
Once infected with C difficile, the rate of disease recurrence is 20-40% when using metronidazole and vancomycin antibiotics as first-line therapy. [3] Fidaxomicin is now recommended as first-line treatment due to a lower risk of CDI recurrence. In patients with CDI who develop fulminant colitis, early surgical consultation is crucial.
Etiology
C difficile colitis results from a disruption of the normal bacterial flora of the colon, colonization with C difficile, and release of toxins that cause mucosal inflammation, mucosal damage, and diarrhea.
Risk factors
Antibiotic exposure
The primary risk factor for C difficile colitis is previous exposure to antibiotics; the most commonly implicated agents include the cephalosporins (especially second and third generation), the fluoroquinolones, ampicillin/amoxicillin, and clindamycin. Less commonly implicated antibiotics are the macrolides (ie, erythromycin, clarithromycin, azithromycin) and other penicillins. Agents occasionally reported to cause the disease include aminoglycosides, trimethoprim-sulfamethoxazole, metronidazole, chloramphenicol, tetracycline, imipenem, and meropenem. Even brief exposure to any single antibiotic can cause C difficile colitis. A prolonged antibiotic course or the use of two or more antibiotics increases the risk of disease.
Hospitalized patients who occupy a bed whose previous occupant received antibiotics appear to have an increased risk of CDI. [4] A multicenter retrospective (2010-2015) study of 100,615 pairs of patients who sequentially occupied a given hospital bed found that less than 1% (576 pairs; 0.57%) of subsequent patients developed CDI, regardless of whether they themselves received antibiotics or not. [4] The association was statistically significant (log-rank P < 0.01).
Proton pump inhibitors
A US Food and Drug Administration (FDA) safety communication on February 8, 2012, described a possible association between the use of proton pump inhibitors (PPIs) and the development of Clostridium difficile –associated diarrhea (CDAD). [5] Data were collected from the US Food and Drug Administration’s (FDA's) Adverse Event Reporting System (AERS) and the medical literature for cases of CDAD in patients undergoing treatment with PPIs.
Many of the adverse event reports involved patients who were elderly, had chronic and/or concomitant underlying medical conditions, or were taking broad-spectrum antibiotics that could have predisposed them to developing CDAD. [5] The FDA also reviewed a total of 28 observational studies described in 26 publications. Of these studies, 23 showed a higher risk of CDI or disease, including CDAD, associated with PPI exposure, compared with no PPI exposure.
Other risk factors
Advanced age (>65 y) and hospitalization (particularly sharing a hospital room with an infected patient, intensive care unit stays, and prolonged hospital stays) are known risk factors for infection with C difficile, as are comorbidities such as kidney disease, liver disease, and cardiovascular disease. [6] Exposure to healthcare settings, chemotherapy, impaired humoral immunity, and gastric acid suppression (medications or bypassing gastric acid via enteral feeds) are also risk factors, [7] as well as gastrointestinal surgery, manipulation of the gastrointestinal tract (ie, tube feeding), and inflammatory bowel disease (IBD). [3, 7, 8] Early emergency general surgery has also been associated with a high incidence of CDI, particularly in patients who receive three or more postoperative antibiotics and those who undergo bowel resections. [9] Vitamin D deficiency may also contribute to an increased risk for CDI. [7]
Risk factors for recurrence of CDI include age older than 65 years, immune compromise, severe underlying illnesses, ongoing antibiotic treatments during CDI, and severe CDI on presentation. [10]
Genetics/genomics
Two genome-wide association studies (GWAS) found an association between a common polymorphism in the upstream promoter of the interleukin (IL)-8 gene and an increased risk for both the initial occurrence and the recurrence of CDI. [11, 12] Neutrophil recruitment to the intestine is thought to be coordinated by IL-8, and the polymorphism in the IL-8 promoter is thought to influence the manner in which neutrophils are recruited to the intestines when CDI is present. [11, 12]
An additional study looked more specifically at the regulation of the regenerating islet-derived genes (REG) in IBD. Interestingly, the study found that the activities of all REG genes were upregulated not only in IBD but also in patients with pseudomembranous colitis. [13] The implication from this study is that the function of the REG family of genes is more generalized in response to inflammation. These proteins are involved in injury, repair, and growth in the intestine. Also of interest is that REG proteins in the gut appear to be antimicrobial, with a function similar to lectin. [13]
Toxin A is an enterotoxin that is responsible for the major manifestations of colitis in humans. In a murine model deficient in the neurokinin-1 receptor, protection against inflammation from toxin A was demonstrated. [14] This protein, which is encoded by the NK1R gene, functions as the receptor for substance P. Downstream effects from this protective effect included decreased intestinal levels of tumor necrosis factor (TNF)-alpha and leukocyte myeloperoxidase. The overall suggestion from this study is that the substance P receptor is very important in the pathogenesis of inflammatory diarrhea. [14]
Further insight into the genetics of C difficile toxin A reveals that the main binding protein is gp96. [15] In addition, it has been found that C difficile has potent stimulatory activity for the Nod1 gene, and mice who were homozygous knockouts for Nod1 had increased lethality to CDI despite similar levels of intestinal damage relative to control the mice. [16] Those mice also had impaired clearance of bacteria and increased translocation of the bacteria. The implication of this study is that Nod1 regulates the susceptibility to C difficile. [16]
Ultimately, many of the genetic influences on CDI and the clinical course of C difficile colitis likely remain unknown. At this point, it is understood that subtle differences in the immune system may significantly influence the natural history of C difficile disease.
Pathophysiology
C difficile colitis results from a disturbance of the normal bacterial flora of the colon, colonization with C difficile, and release of toxins that cause mucosal inflammation and damage. Colonization occurs by the fecal-oral route. Hospitalized patients are the primary targets of CDI, although C difficile is present as a colonizer in 2-3% of healthy adults and in as many as 70% of healthy infants. [17] (Treatment of asymptomatic carriers is not recommended.)
C difficile forms heat-resistant spores that can persist in the environment for several months to years. Outbreaks of C difficile diarrhea may occur in hospitals and outpatient facilities where contamination with spores is prevalent. Although the normal gut flora resists colonization and overgrowth with C difficile, the use of antibiotics, which alter and suppress the normal flora, allows proliferation of C difficile, toxin production, and diarrhea.
Pathogenic strains of C difficile produce two distinct toxins. Toxin A is an enterotoxin, and toxin B is a cytotoxin; both are high–molecular weight proteins capable of binding to specific receptors on the intestinal mucosal cells. Receptor-bound toxins gain intracellular entry by catalyzing a specific alteration of Rho proteins—small glutamyl transpeptidase (GTP)–binding proteins that assist in actin polymerization, cytoskeletal architecture, and cell movement. Both toxin A and toxin B appear to play a role in the pathogenesis of C difficile colitis in humans.
More recently, rat studies suggest that C difficile toxin B induces senescence in enteric glial cells (EGCs); investigators hypothesize that EGCs that survive toxin B and acquire senescence potentially cause the development of irritable bowel syndrome and inflammatory bowel disease via persistent inflammation, transfer of senescence status, and stimulation of preneoplastic cells. [18]
The NAP1 hypervirulent strain of C difficile is associated with the most serious sequelae of CDI, causing severe and fulminant colitis that is characterized by leukocytosis, renal failure, and toxic megacolon. [19] The widespread use of fluoroquinolone antibiotics may have played a role in the proliferation of the NAP1 strain. Once rising white blood cell count or hemodynamic instability occurs and fulminant colitis is imminent, subtotal colectomy with end ileostomy is often necessary. Fecal bacteriotherapy and immunotherapy are investigative treatment strategies that have potential for managing patients with severe CDI. [19]
Epidemiology
Occurrence in the United States
In the United States, CDI occurs primarily in hospitalized patients, causing as many as 3 million cases of diarrhea and colitis per year. Cancer patients are significantly affected by CDI healthcare-associated diarrhea. [20] Diarrhea caused by C difficile is also linked to 14,000 American deaths annually. [21]
The incidence of reported CDI infection continues to increase. In the 1980s, McFarland et al reported that 7% of patients admitted to a hospital and 28% of patients who were hospitalized had positive cultures for the organism. By the 1990s, the incidence of C difficile in hospitalized patients had risen to 30-40 per 100,000 population, and by 2005, to 84 per 100,000 population.
Indeed, in contrast to the incidence rates of other nosocomial infections, which declined from 2000 to 2009, the number of hospitalized patients with any CDI as a discharge diagnosis more than doubled in the same period, from approximately 139,000 to 336,600. Furthermore, the number of patients with a primary diagnosis of CDI more than tripled, from 33,000 to 111,000. [22]
New CDI populations have emerged, and studies have challenged the notion that C difficile is primarily a hospital infection, as more cases are being seen in the community. [2, 23, 24] These cases include patients with community-acquired infection and no previous antibiotic exposure, pregnant women, and patients with inflammatory bowel disease (IBD). [3, 8]
A population-based study from Olmsted County, Minnesota, demonstrated that 41% of the cases of CDI were community acquired and that the incidence of both community- and hospital-acquired C difficile increased significantly from 1991 to 2005. [2]
International occurrence
The incidence of CDI, as well as deaths attributable to C difficile, has also risen in Europe and Canada. In Canada's Estrie region of Quebec, the incidence quadrupled in 2003 to 92.2 cases per 100,000 population. In a survey of 97 hospitals across 34 European countries, the incidence of C difficile in hospitalized patients was 41 per 100,000 patient-days. [25]
The worldwide increased incidence of CDI has been attributed to a variety of risk factors, including more elderly patients in the population, treatment resistance to fluoroquinolones, and the emergence of a newly discovered, more virulent strain of C difficile (BI/NAP1/027). [26] Additional risk factors toward increasing CDIs include the use of penicillins and clindamycin, as well as an increased use in the total number of antibiotics in the community. [26]
Age-related demographics
CDI is more common in elderly people, and old age may promote susceptibility to colonization and disease. Cross-infection by C difficile is common in neonatal units, but neonates do not seem to develop C difficile –associated diarrhea. More recently, there have been specific populations affected by C difficile that were previously believed to be at low risk, such as young, healthy persons not exposed to a hospital environment or antimicrobial therapy and young women in a peripartum setting.
A study by Nylund et al suggested an increase in CDI among hospitalized children, especially in those with medical conditions such as IBD and immunosuppression. [27] Also at risk are individuals hospitalized with conditions that require antibiotic administration.
Prognosis
Some patients with C difficile colitis with mild disease may recover without specific therapy; however, persistent diarrhea may be debilitating and can last for several weeks; therefore, treatment is recommended even in mild disease
The use of oral metronidazole or vancomycin produces response rates of greater than 95% in mild to moderate cases, with symptomatic improvement (diarrhea) in as little as 3-4 days and complete resolution in 7-10 days. Yet approximately 20-27% of patients treated for a first episode of C difficile colitis relapse after successfully completing therapy, typically 3 days to 3 weeks after treatment has ended. Patients who relapse once are at an even greater risk for further relapses; the relapse rate for patients with 2 or more relapses is 65%. [28]
Adverse outcomes from CDI include treatment failure, development of severe or severe-complicated infection, sepsis and the need for admission to the intensive care unit, need for colectomy, increased length of hospital stay, need for hospital admission in patients with community-acquired CDI, and mortality. [29] (Mortality from C difficile –associated diarrhea as high as 4.2-6.9% were found in several centers in North America; in a multicenter study from Quebec, mortality increased with age. [30] )
However, there is evidence that reliance primarily on hospital-based studies of CDI, with relatively few studies having been made of community-acquired cases, may have led researchers to underestimate the burden, but overestimate the severity, of the condition. [2] Patients with community-acquired CDI are generally younger, with less severe infection relative to hospitalized patients with CDI. [2]
Severe strains
Several outbreaks have been caused by the North American Pulsed Field type 1 and polymerase chain reaction (PCR) ribotype 027 (NAP1/027) strain. This virulent strain has been associated with increased production of toxins A and B, fluoroquinolone resistance, and the production of a binary toxin. The role of the binary toxin is not clear, but it may synergistically increase the virulence of toxins A and B.
Based on the data from the Centers of Disease Control and Prevention (CDC), the virulent strain NAP1/027 has been reported in most US states and in several countries in Europe.
PCR ribotypes 018 and 056, identified in Europe, have been associated with more severe Cdifficile colitis. [25]
Predictors of severe outcomes in IBD and ulcerative colitis
Patients with IBD are well known to be at an increased risk for CDI. A study identified 3 independent predictors for severe outcomes in hospitalized IBD patients with CDI: (1) serum albumin less than 3 g/dL, (2) hemoglobin level below 9 g/dL, and (3) elevated serum creatinine above 1.5 mg/dL. [31]
In another study, investigators found that CDI is associated with a worse long-term outcome in patients with ulcerative colitis. [32] In the year following treatment for CDI in these patients, an escalation in medical management was noted. In addition, independent predictors for colectomy within 1 year were infection with C difficile and endoscopically proven severe disease. [32]
Complications
Fulminant colitis
Fulminant colitis is a rare form of CDI, occurring in only 3% of patients but accounting for most of the serious complications. These include toxic megacolon, colonic perforation, and death. Surgical intervention is usually required in patients who develop fulminant colitis.
Sailhamer et al conducted a retrospective review of 4796 patients with C difficile colitis, 199 (4.1%) of whom had the fulminant form, as defined by the need for colectomy or admission to the intensive care unit. [33] The in-hospital mortality for fulminant C difficile colitis was 34.7%. The investigators determined that independent predictors of mortality included the following:
-
Age 70 years or older
-
Severe leukocytosis or leukopenia or bandemia
-
Cardiorespiratory failure
The presence of all 3 factors resulted in a 57.1% mortality; in the absence of all 3, the mortality was 0%. [33] The investigators concluded that despite awareness and treatment of fulminant C difficile colitis, this condition remains highly lethal. Thus, reliable predictors of mortality should be used to prompt aggressive surgical intervention. [33]
To determine the long-term survival, rate of gastrointestinal continuity restoration, and rate of recurrence following an attack of fulminant C difficile colitis, Miller et al searched a pathologic database for patients with this condition, defined as those who had a bout of C difficile colitis and whose disease required surgical intervention after failure of medical therapy. [34]
Of the 49 patients who fit the criteria, the investigators found a 30-day mortality of 57% (28/49), with an in-hospital mortality of 49%. Moreover, the 5-year survival for the long-term survival group was poor, at 38% (16.3% for all patients). Twenty percent of patients had restored gastrointestinal continuity. One case of recurrence of C difficile colitis was reported. [34]
Toxic megacolon
Toxic megacolon is an acute toxic colitis with dilatation of the colon. This condition is diagnosed clinically in a patient with signs and symptoms of severe toxicity, the presence of a tender abdomen, and a dilated colon on plain radiograph of the abdomen.
Colonic perforation
Colonic perforation is usually accompanied by abdominal rigidity, involuntary guarding, rebound tenderness, and absent bowel sounds. Free air may be revealed on abdominal radiographs. Any suspicion of perforation in this setting should prompt immediate surgical consultation.
-
Clostridioides (Clostridium) Difficile Colitis. Endoscopic visualization of pseudomembranous colitis, a characteristic manifestation of full-blown C difficile colitis. Classic pseudomembranes are visible as raised, yellow plaques ranging from 2 to 10 mm in diameter and scattered over the colorectal mucosa. Courtesy of Gregory Ginsberg, MD, University of Pennsylvania.
-
Clostridioides (Clostridium) Difficile Colitis. Colonic pseudomembranes of pseudomembranous colitis. Photographs courtesy of Eric M Osgard, MD.
-
Clostridioides (Clostridium) Difficile Colitis. Gross pathology specimen from a case of pseudomembranous colitis revealing the characteristic yellowish plaques.
-
Clostridioides (Clostridium) Difficile Colitis. Gross pathology specimen from a case of pseudomembranous colitis, again demonstrating the characteristic yellowish plaques.
-
Clostridioides (Clostridium) Difficile Colitis. Frontal abdominal radiograph in a patient with proven pseudomembranous colitis. Note the nodular haustral thickening, most pronounced in the transverse colon.
-
Clostridioides (Clostridium) Difficile Colitis. Barium enema demonstrating the typical serrated appearance of the barium column (resulting from trapped barium between the edematous mucosal folds and the plaquelike membranes of pseudomembranous colitis).
-
Clostridioides (Clostridium) Difficile Colitis. Axial computed tomography scan of pseudomembranous colitis.
-
Clostridioides (Clostridium) Difficile Colitis. Computed tomography scan depicting pseudomembranous colitis.
-
Clostridioides (Clostridium) Difficile Colitis. Ultrasonographic image of pseudomembranous colitis.






