Air - Thermophysical Properties
Thermal properties of air at different temperatures - density, viscosity, critical temperature and pressure, triple point, enthalpi and entropi, thermal conductivity and diffusivity and more.
Thermophysical properties of air:
- Boiling temperature (at 1 bara): 78.8 K = -194.4 °C = -317.8 °F
- Bulk modulus elasticity : 1.01325 x 105 Pa or N/m2
- Condensation temperature (at 1 bara): 81.8 K = -191.4 °C = -312.5 °F
- Critical temperature : 132.63 K = -140.52 °C = -220.94 °F
- Critical pressure : 37.363 atm = 37.858 bar = 3.7858 MPa (MN/m2) = 549.08 psi (=lbf /in2)
- Critical density: 10.448 mol/dm3 = 302.6 kg/m3 = 0.5871 slug/ft3 = 18.89 lbm /ft3
- Density (at 0°C and 1 bara): 1.276 kg/m3 = 0.00248 slug/ft3 = 0.0797 lb/ft3
- Density (at 60°F and 1 atm): 1.208 kg/m3 = 0.00234 slug/ft3 = 0.0754 lb/ft3
- Enthalpy (heat) of air at 0°C and 1 bara: 11.57 kJ/mol = 399.4 kJ/kg = 171.7 Btu(IT)/lb
- Entropy of air at 0°C and 1 bara: 0.1100 kJ/mol K = 3.796 kJ/kg K = 0.9067 Btu(IT)/lb °F
- Liquid density at boiling point and 1 bar: 875.50 kg/m3 = 54.656 lb/ft3
- Molar mass: 28.9647 g/mol
- Specific heat capacity (Cp ) air at 0°C and 1 bara: 1.006 kJ/kgK = 0.24028 Btu(IT)/(lbm °F) or kcal/(kg K)
- Specific heat capacity (Cv ) air at 0°C and 1 bara: 0.7171 kJ/kgK = 0.17128 Btu(IT)/(lbm °F) or kcal/(kg K)
- Thermal conductivity at 0°C and 1 bara: 24.35 mW/(m K) = 0.02094 kcal(IT)/(h m K) = 0.01407 Btu(IT)/(h ft °F)
- Thermal expansion coefficient at 0°C and 1 bara: 0.00369 1/K = 0.00205 1/°F
- Triple point pressure: 0.05196 atm = 0.05265 bar = 5265 Pa = 0.7636 psi (=lbf /in2)
- Triple point temperature: 59.75 K = -213.40 °C = -352.12 °F
- Viscosity, dynamic , at 0°C and 1 bara: 17.22 μPa s = 0.01722 cP = 0.3596x 10-6 ( lbf s)/ft2= 11.57 x10-6 lbm /(ft s)
- Viscosity, kinematic , at 0°C and 1 bara: 0.00001349 m2/s = 13.49 cSt = 0.0001452 ft2/s
Follow the links below to get values for the listed properties of air at varying pressure and temperature :
- Density at varying pressure
- Density, specific weight and thermal expansion coefficient at varying temperature
- Diffusion coefficients of gases in excess of air
- Dynamic and kinematic viscosity
- Prandtl's number
- Properties at gas-liquid equilibrium condition
- Specific heat (heat capacity) at varying pressure
- Specific heat (heat capacity) at varying temperature
- Thermal conductivity
- Thermal diffusivity
See also more about atmospheric pressure , and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure ,
as well as Thermophysical properties of: Acetone , Acetylene , Ammonia , Argon , Benzene , Butane , Carbon dioxide , Carbon monoxide , Ethane , Ethanol , Ethylene , Helium , Hydrogen , Hydrogen sulfide , Methane , Methanol , Nitrogen , Oxygen , Pentane , Propane , Toluene , Water and Heavy water, D2O .
Air is a mixture of gases at standard conditions. However, at low temperature and high pressures the gas mixture becomes a liquid. The phase diagram for air shows the phase behavior with changes in temperature and pressure. The curve between the triple point and the critical point shows the air boiling point with changes in pressure.
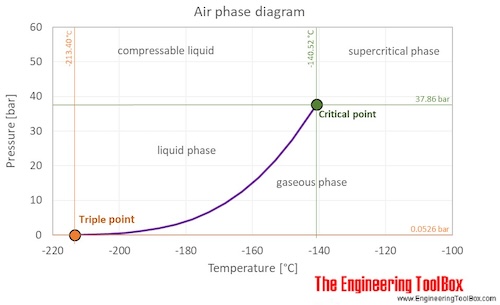
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.
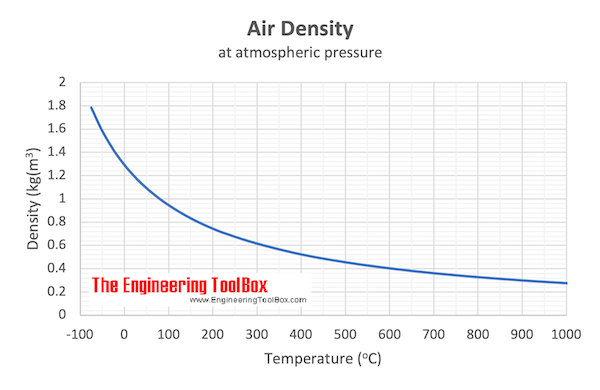
Download and print Air - Density vs. Temperature Chart
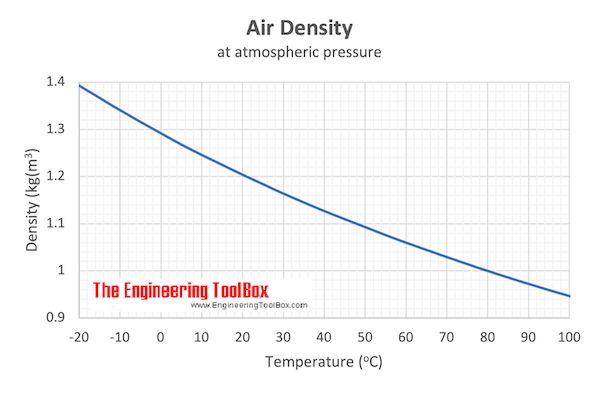
Download and print Air - Density vs. Temperature Chart
Example - Mass of Air at Temperature 100 oC
From the table above - the density of air is 0.946 kg/m3 at 100 oC . The mass of 10 m 3 air can be calculated as
m = V ρ
= (10 m3 ) (0.946 kg/m3 )
= 9.46 kg
where
m = mass (kg)
V = volume (m3 )
ρ = density (kg/m3 )
Example - Mass of Air at Temperature 20 oC
From the table above - the density of air is 1.205 kg/m3 at 20 oC . The mass of 10 m 3 air can be calculated as
m = (10 m3 ) (1.205 kg/m3 )
= 12.05 kg
Example - Lifting Force of a Hot Air Balloon
An air balloon with volume 10 m3 is heated to 100 oC . The temperature of the surrounding air is 20 oC. The change in gravity force (weight) of the air volume is the potential lifting force of the balloon. The lifting force can be calculated as
F l = dm a g
= V d ρ a g
= (10 m3 ) [(1.205 kg/m3 ) - (0.946 kg/m3 )] (9.81 m/s2)
= 25.4 N
where
F l = lifting force - change in gravity force (weight) (N)
a g = acceleration of gravity (9.81 m/s2)
dm = V d ρ = change of mass in the balloon (kg)
dρ = change in density due to temperature difference (kg/m3 )
Related Topics
-
Air Psychrometrics
Moist and humid air calculations. Psychrometric charts and Mollier diagrams. Air-condition systems temperatures, absolute and relative humidities and moisture content in air. -
Basics
Basic engineering data. SI-system, unit converters, physical constants, drawing scales and more. -
Densities
Densities of solids, liquids and gases. Definitions and convertion calculators. -
Environment
Climate, meteorology, solar, wind, emissions and environmental related engineering resources. -
Fluid Mechanics
The study of fluids - liquids and gases. Involving velocity, pressure, density and temperature as functions of space and time. -
Gases and Compressed Air
Properties of air, LNG, LPG and other common gases. Pipeline capacities and sizing of relief valves. -
Material Properties
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more. -
Thermodynamics
Work, heat and energy systems. -
Viscosities
Viscosities of products and chemical species at varying conditions.
Related Documents
-
Acetone - Thermophysical Properties
Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included. -
Air - Composition and Molecular Weight
Dry air is a mechanical mixture of nitrogen, oxygen, argon and several other gases in minor amounts. -
Air - Density and Specific Volume vs. Altitude
Density and specific volume of air varies with elevation above sea level. -
Air - Density vs. Pressure and Temperatures
Air density at pressure ranging 1 to 10 000 bara (14.5 - 145000 psi) and constant selected temperatures. -
Air - Density, Specific Weight and Thermal Expansion Coefficient vs. Temperature and Pressure
Online calculator, figures and tables showing density, specific weight and thermal expansion coefficients of air at temperatures ranging -100 to 1600 °C (-140 to 2900 °F) at atmospheric and higher pressure - Imperial and SI Units. -
Air - Diffusion Coefficients of Gases in Excess of Air
Diffusion coefficients (D12) for gases in large excess of air at temperatures ranging 0 - 400 °C. -
Air - Dynamic and Kinematic Viscosity
Online calculator, figures and tables with dynamic (absolute) and kinematic viscosity for air at temperatures ranging -100 to 1600°C (-150 to 2900°F) and at pressures ranging 1 to 10 000 bara (14.5 - 145000 psia) - SI and Imperial Units. -
Air - Molecular Weight and Composition
Dry air is a mixture of gases where the average molecular weight (or molar mass) can be calculated by adding the weight of each component. -
Air - Prandtl Number
Prandtl number for air vs. temperature and pressure. -
Air - Properties at Gas-Liquid Equilibrium Conditions
Properties of air change along the boiling and condensation curves (temperature and pressure between triple point and critical point conditions). An air phase diagram included. -
Air - Specific Heat vs. Pressure at Constant Temperature
Figures and tables with isobaric (Cp) and isochoric (Cv) specific heat of air at constant temperature and pressure ranging 0.01 to 10000 bara. -
Air - Specific Heat vs. Temperature at Constant Pressure
Online calculator with figures and tables showing specific heat (Cp and Cv) of dry air vs. temperature and pressure. SI and imperial units. -
Air - Speed of Sound vs. Temperature
Speed of sound in air at standard atmospheric pressure with temperatures ranging -40 to 1000 °C (-40 to 1500 °F) - Imperial and SI Units. -
Air - Thermal Conductivity vs. Temperature and Pressure
Online calculator with figures and tables showing air thermal conductivity vs. temperature and pressure. SI and imperial units. -
Air - Thermal Diffusivity vs. Temperature and Pressure
Figures and tables withdry air thermal diffusivity vs. temperarure and pressure. SI and Imperial units. -
Air - Volume vs. Temperature
Air volume vs. temperature correction factor compared to Normal Air Conditions. -
Air Cooling of Storage Roooms
Heat removed from storage rooms with cooled air. -
Air Properties - Imperial Units
Thermodynamic properties of air at low pressures - imperial units. -
Air Properties - SI Units
Ideal gas properties of air at low pressure - SI units. -
Air Temperature, Pressure and Density vs. Altitude
Elevation above sea level and air temperature, pressure and density. -
Ammonia - Thermophysical Properties
Chemical, Physical and Thermal Properties of Ammonia. Phase diagram included. -
Benzene - Thermophysical properties
Chemical, physical and thermal properties of benzene, also called benzol. Phase diagram included. -
Carbon dioxide - Prandtl Number vs. Temperature and Pressure
Figures and table with changes in Prandtl number for carbon dioxide with changes in temperature and pressure. -
Carbon Dioxide - Thermophysical Properties
Chemical, physical and thermal properties of carbon dioxide. Phase diagram included. -
Dry Air - Thermodynamic and Physical Properties
Thermodynamic properties of dry air - specific heat, ratio of specific heats, dynamic viscosity, thermal conductivity, Prandtl number, density and kinematic viscosity at temperatures ranging 175 - 1900 K. -
Dry Air and Water Vapor - Density and Specific Volume vs. Temperature - Imperial Units
Density and specific volume of dry air and water vapor at temperatures ranging 225 to 900 degF (107 to 482 degC). -
Ethane - Thermophysical Properties
Chemical, Physical and Thermal Properties of Ethane - C2H6. -
Ethylene - Thermophysical Properties
Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas. Phase diagram included. -
Global Temperature Trend
Global temperature trend year 1880 - 2015. -
Heavy Water - Thermophysical Properties
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more. -
Helium - Thermophysical Properties
Chemical, Physical and Thermal Properties of Helium - He. -
Hot Air Balloons - Calculate Lifting Weights
Calculate hot air ballons lifting forces. -
Humid Air - Heating
Enthalpy change and temperature rise when heating humid air without adding moisture. -
Liquid Ammonia - Thermal Properties at Saturation Pressure
Density, specific heat, thermal conductivity, viscosity and Prandtls no. of liquid ammonia at saturation pressure. -
Liquids - Volumetric Expansion Coefficients
Volumetric - or cubical - expansion coefficients for common liquids. -
Methane - Thermophysical Properties
Chemical, Physical and Thermal Properties of Methane - CH4. Phase diagram included. -
Methanol - Thermophysical Properties
Chemical, physical and thermal properties of methanol, CH3OH (also called carbinol, wood alcohol, hydroxy methyl and methyl alcohol). Phase diagram included. -
Mixing of Humid Air
The change in state wwhen mixing moist air - enthalpy, heat, temperature and specific humidity. -
Moist Air - Density vs. Pressure
Density of moist air vs. pressure ranging 75 - 1000 mmHg. -
Moist Air - Enthalpy
Sensible and latent heat of moist air. -
Moist Air - Psychrometric Table for Pressure 29.92 inHg
Dry and wet bulb temperatures, saturation pressure, water vapor weight, specific volume, heat and more. -
Pentane - Thermophysical Properties
Chemical, physical and thermal properties of pentane, also called n-pentane. Phase diagram included. -
Removing Heat with Air
Calculating heat removed with air by measuring the wet bulb temperature. -
Room Sensible Heat Factor - RSHF
Room Sensible Heat Factor - RSHF - is defined as the sensible heat load divided by the total heat load in a room -
Storing Thermal Heat in Materials
Energy stored as sensible heat in materials. -
STP - Standard Temperature and Pressure and NTP - Normal Temperature and Pressure
The definition of STP - Standard Temperature and Pressure and NTP - Normal Temperature and Pressure. -
Thermal Conductivity - Online Converter
Convert between thermal conductivity units. -
Thermodynamic Terms - Functions and Relations
Common thermodynamic terms and functions - potential energy, kinetic energy, thermal or internal energy, chemical energy, nuclear energy and more. -
Third Law of Thermodynamics
The entropy of a substance is zero if the absolute temperature is zero. -
Universal and Individual Gas Constants
The Universal and Individual Gas Constants in fluid mechanics and thermodynamics. Individual gas constants for the most common gases. -
Viscosity - Absolute (Dynamic) vs. Kinematic
Vicosity is a fluid's resistance to flow and can be valued as dynamic (absolute) or kinematic. -
Volumetric (Cubic) Thermal Expansion
Volumetric temperature expansion calculator. -
Wind Power
Power generation from wind.




