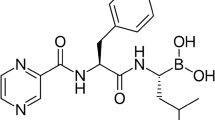
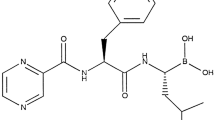
Summary
Abstract
Bortezomib (VELCADE®) is a proteasome inhibitor that not only targets the myeloma cell, but also acts in the bone marrow micro-environment, inhibiting the binding of myeloma cells to bone marrow stromal cells, as well as demonstrating anabolic effects on bone.
Intravenous bortezomib, with or without dexamethasone, is effective and well tolerated in patients with relapsed/refractory multiple myeloma, as demonstrated in the phase II CREST and SUMMIT trials, and the phase III APEX trial, and is a recommended treatment for this patient group. Based on the results of another phase III trial, the combination of bortezomib plus pegylated liposomal doxor-ubicin is also a recommended treatment for patients with relapsed/refractory multiple myeloma. Other bortezomib-combination regimens have demonstrated promising response data in phase II trials in patients with relapsed/refractory disease, although response and survival data for these combinations need to be confirmed in larger phase III trials.
Bortezomib was effective and well tolerated when used as part of a first-line regimen in previously untreated patients with multiple myeloma. In the phase III VISTA trial in elderly patients with previously untreated multiple myeloma not eligible for transplantation, bortezomib in combination with melphalan and prednisone was effective and well tolerated and is a recommended treatment regimen for this group of patients. Preliminary data from phase II/III trials in patients with previously untreated multiple myeloma indicate a promising role for the use of bortezomib combined with various other chemotherapeutic agents as induction therapy prior to transplantation.
Pharmacological Properties
Bortezomib is a modified dipeptidyl boronic acid analogue that binds selectively and reversibly to the 26S proteasome. Inhibition of the 26S proteasome prevents the degradation of key proteins and affects multiple signalling cascades within the cell, ultimately leading to cell death. Bortezomib acts in the bone marrow micro-environment by inhibiting the binding of myeloma cells to bone marrow stromal cells. Bortezomib also has an anabolic effect on bones, inhibiting human osteoclast activity and stimulating osteoblast function. Bortezomib is cytotoxic to a number of in vitro cancer cells and delays tumour growth in nonclinical models of cancer, including multiple myeloma. In clinical studies, maximum inhibition of 20S proteasome activity occurred within 1 hour of bortezomib administration, after which inhibition slowly declined and returned to baseline by 72 hours.
Following multiple intravenous doses of bortezomib 1.3mg/m2, mean maximum plasma concentrations (89–120 ng/mL) were reached quickly (≤0.20 hours). Bortezomib is distributed widely to peripheral tissues and is extensively bound to human plasma proteins (mean 83%). Bortezomib is primarily metabolized by the cytochrome P450 (CYP) enzymes CYP3A4, CYP2C19 and CYP1A2, with a minor amount of metabolism occurring via CYP2D6 and CYP2C9. Bortezomib 1.3 mg/m2 was eliminated more rapidly after the first dose than after subsequent doses (elimination half-life 12 vs 76–108 hours).
Therapeutic Efficacy
Two open-label, phase II trials (SUMMIT [n = 202] and CREST [n = 54]) established the efficacy of bortezomib 1.3mg/m2 (with or without dexa-methasone) administered by intravenous bolus on days 1, 4, 8 and 11 of a 21-day cycle for a maximum of eight cycles in heavily pretreated patients with relapsed/refractory multiple myeloma. With this regimen, the percentages of patients with at least a minimal response (≥MR; i.e. complete [CR] plus partial [PR] plus MR) was 50% in CREST and 35% in SUMMIT. The randomized, open-label, phase III APEX trial which randomized 669 patients with relapsed multiple myeloma after one to three previous therapies demonstrated the superiority of a bortezomib 1.3 mg/m2 regimen over a high-dose dexa-methasone regimen. As a result, the high-dose dexamethasone arm was halted at a planned interim analysis and all dexamethasone recipients were offered bortezomib. The median time to disease progression (TTP) was longer (6.2 vs 3.5 months; p< 0.001) with bortezomib than dexamethasone. With the bortezomib regimen, more patients achieved ≥PR (38% vs 18%; p<0.001) and CR (6% vs <1%; p<0.001), and the overall 1-year survival rate was higher (80% vs 66%; p = 0.003).
The combination of bortezomib 1.3 mg/m2 plus pegylated liposomal doxo-rubicin significantly prolonged the TTP compared with bortezomib alone in a phase III trial in 646 bortezomib-naive patients with relapsed or refractory multiple myeloma. Bortezomib plus pegylated liposomal doxorubicin versus bortezomib alone was associated with a 45% reduction in the risk of disease progression; median TTP was 6.5 months with bortezomib alone and increased to 9.3 months with the combination regimen (p = 0.000004). The ≥PR rates were not significantly different between the two treatment arms of the trial; however, the median duration of response was longer with the combination regimen (10.2 vs 7.0 months; p = 0.0008). When thalidomide was added to a regimen of bortezomib plus pegylated liposomal doxorubicin (using a 28-day cycle), the ≥PR rate was 65% and CR rate was 23%.
Phase I/II trials in patients with relapsed/refractory disease have demonstrated that various three- or four-drug combinations involving bortezomib and other agents (including melphalan, prednisone, cyclophosphamide, dexamethasone, thalidomide or lenalidomide) were associated with promising responses (≥PR rates of 66–92%) and survival data. These combinations have yet to be fully investigated in phase III trials.
A regimen of bortezomib plus melphalan plus prednisone was significantly more effective than a regimen of melphalan plus prednisone with respect to the TTP and response rates, according to data from the phase III VISTA trial in 682 elderly patients with newly diagnosed multiple myeloma who were ineligible for transplantation. The bortezomib-containing regimen was associated with a 52% reduction in the risk of disease progression; median TTP was 24.0 versus 16.6 months, respectively (p< 0.001). The ≥PR rates (71% vs 35%) and CR rates (30% vs 4%) were also significantly higher (both p< 0.001) with the bortezomib-containing regimen. After a median follow-up of 25.9 months, the bortezomib-containing regimen was associated with higher 3-year survival rates (72% vs 59%; p = 0.003). Preliminary data from a phase III trial in 354 patients aged >65 years with newly diagnosed disease indicated that the addition of thalidomide to the bortezomib-containing regimen used in the VISTA trial was associated with an improved response (at least a very good partial response [≥VGPR] 55% vs 45%; p = 0.02).
Preliminary data from phase III trials have demonstrated the efficacy of induction regimens of bortezomib plus dexamethasone (≥VGPR 39%), bortezomib plus dexamethasone plus thalidomide (≥VGPR 62%) and bortezomib plus doxorubicin plus dexamethasone (≥VGPR 42%) in patients with newly diagnosed multiple myeloma. Bortezomib-containing induction regimens were superior to the comparator regimens for overall response rates (≥PR) both before and after autologous stem cell transplantation. Preliminary data from phase I/II trials have indicated high activity (≥VGPR of 55–74%) with various other bortezomib-containing induction regimens.
Tolerability
The most frequently reported adverse events (incidence ≥30%) associated with the use of bortezomib are asthenic conditions (fatigue, weakness, malaise), gastrointestinal events (nausea, diarrhoea, constipation, vomiting), peripheral neuropathy, pyrexia, thrombocytopenia, neutropenia, psychiatric disorders and anorexia/decreased appetite, according to data from the phase III APEX trial in patients with refractory or relapsed multiple myeloma. In the APEX trial, grade 3 or 4 adverse events occurred in 61% and 14% of bortezomib recipients. Common grade 3 adverse events associated with bortezomib included thrombocytopenia and neutropenia (26% and 12%); these adverse events were also the most commonly reported grade 4 adverse events (4% and 2%). Peripheral neuropathy occurred in 36% of bortezomib recipients in the APEX trial (7% with grade 3 and 1% with grade 4). Improvement or resolution of at least grade 2 peripheral neuropathy occurred in 64% of bortezomib recipients, with a median time to resolution of 110 days from onset. A change in the dose or schedule of administration of bortezomib is recommended in patients who experience new or worsening peripheral neuropathy.
In general, the combination of bortezomib with other chemotherapeutic agents resulted in a tolerability profile that was consistent with the known tolerability profiles of the individual agents involved, and did not result in any unexpected adverse events, according to data from phase III trials. Drug-related adverse events could generally be managed by dosage modifications and supportive therapy.









Similar content being viewed by others
References
National Comprehensive Cancer Network. Practise Guidelines in Oncology: multiple myeloma [online]. Available from URL: http://www.nccn.org [Accessed 2009 Jan 8]
Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med 2004; 351(18): 1860–73
Jemal A, Siegel R, Ward E. Cancer statistics, 2008. CA Cancer J Clin 2008; 58(2): 71–96
Harousseau J-L, Dreyling M, ESMO Guidelines Working Group. Multiple myeloma: ESMO clinical recommendations for diagnosis, treatment and follow-up. ESMO Guidelines Working Group. Ann Oncol 2008; 19 Suppl. 2: ii55–7
Brenner H, Gondos A, Pulte D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006–2010. Haematologica 2009; 94(2): 270–5
International Myeloma Foundation. Multiple myeloma: concise review of the disease and treatment options (2008/2009) [online]. Available from URL: http://www.myeloma.org [Accessed 2009 Jan 20]
Shah SR, Tran TM. Lenalidomide in myelodysplastic syndrome and multiple myeloma. Drugs 2007; 67(13): 1869–81
Millennium Pharmaceuticals Inc. Velcade (bortezomib) for injection: US prescribing information [online]. Available from URL: http://www.fda.gov/cder/foi/label/2008/021602s015lbl.pdf [Accessed 2008 Oct 23]
Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumour agents. Can Res 1999; 59(11): 2615–22
Sterz J, von Metzer I, Hahne JC, et al. The potential of proteasome inhibitors in cancer therapy. Expert Opin Invest Drugs 2008 Jun; 17(6): 879–95
Cayrol C, Ducommun B. Interaction with cyclin-dependent kinases and PCNA modulates proteasome-dependent degradation of p21. Oncogene 1998; 17(19): 2437–44
Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 2001; 61(7): 3071–6
Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p531. Cancer Res 1996; 56(11): 2649–54
Marshansky V, Wang X, Bertrand R, et al. Proteasomes modulate balance among proapoptotic and antiapoptotic Bcl-2 family members and compromise functioning of the electron transport chain in leukemic cells. J Immunol 2001; 166(5): 3130–42
Stancovski I, Gonen H, Orian A, et al. Degradation of the proto-oncogene product c-Fos by the ubiquitin proteolytic system in vivo and in vitro: identification and characterization of the conjugating enzymes. Mol Cell Biolo 1995 Dec; 15(12): 7106–16
Kho CJ, Huggins GS, Endege WO, et al. Degradation of E2A proteins through a ubiquitin-conjugating enzyme, UbcE2A. J Biol Chem 1997; 272(6): 3845–51
Chang YC, Lee YS, Tejima T, et al. Mdm2 and bax, downstream mediators of the p53 response, are degraded by the ubiquitin-proteasome pathway. Cell Growth Differ 1998; 9: 79–84
Traenckner EB-M, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-κB and stabilizes a newly phosphorylated form of IκB-α that is still bound to NF-κB. EMBO J 1994 Nov; 13(22): 5433–41
Chen C, Edelstein LC, Gelinas C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol Cell Biol 2000; 20(8): 2687–95
Takahashi S, Harigae H, Ishii KK, et al. Over-expression of Flt3 induces NF-kappaB pathway and increases the expression of Il-6. Leuk Res 2005; 29: 893–9
Strauss SJ, Higginbottom K, Juliger S, et al. The proteasome inhibitor bortezomib acts independently of p53 and induces cell death via apoptosis and mitotic catastrophe in B-cell lymphoma cell lines. Cancer Res 2007 Mar 15; 67(6): 2783–90
Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood 2003 Mar 13; 101(6): 2377–80
Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res 2003; 9(3): 1136–44
Yan H, Wang YC, Li D. Arsenic trioxide and proteasome inhibitor bortezomib synergistically induces apoptosis in leukemia cells: the roll of protein kinase Cδ. Leukemia 2007; 21(7): 1488–95
Teicher BA, Ara G, Herbst R. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res 1999 Sep; 5(9): 2638–45
Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol 2002; 20(22): 4420–7
Stewart AK, Sullivan D, Lonial S, et al. Pharmacokinetic and pharmacodynamics study of two doses of bortezomib in patients with relapsed multiple myeloma [abstract no. 3533]. Blood 2006; 108 (11 Pt I): 1008a
Pennisi A, Li X, Ling W, et al. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol 2009; 84(1): 6–14
Terpos E. Bortezomib directly inhibits osteoclast function in multiple myeloma: implications into the management of myeloma bone disease. Leuk Res 2008 Nov; 32(11): 1646–7
Terpos E, Sezer O, Croucher P. Myeloma bone disease and proteasome inhibition therapies. Blood 2007; 110(4): 1098–104
von Metzler I, Krebbel H, Hecht M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia 2007 Sep; 21(9): 2025–34
Giuliani N, Morandi F, Tagliaferri S, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 2007; 110(1): 334–8
Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol 2006 Dec; 135(5): 688–92
Terpos E, Dimopoulos MA, Sezer O. The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia 2007 Sep; 21(9): 1875–84
Pinzone JJ, Hall BM, Thudi NK, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009; 113(3): 517–25
Heider U, Kaiser M, Müller C, et al. Treatment of bortezomib increases osteoblast function in patients with multiple myeloma. Eur J Haematol 2006; 77(3): 233–8
Zangari M, Esseltine D, Lee C-K, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol 2005 Oct; 131(1): 71–3
Delforge M, Richardson PG, Schlag R, et al. VMP results in fewer bone events and greater ALP increases vs MP in the VISTA study in frontline MM [abstract no. 246]. 12th International Myeloma Workshop; 2009 Feb 26–Mar 1 2009; Washington, DC
Dimopoulos MA, Kastritis E, Rosinol L, et al. Pathogen-esis and treatment of renal failure in multiple myeloma. Leukemia 2008; 22(8): 1485–93
Terpos E, Katodritou E, Triftsakis E. Cystatin-C is an independent prognostic factor for survival in multiple myeloma and is reduced by bortezomib administration. Haematologica 2009; 94(3): 372–9
Ludwig H, Drach J, Graf H, et al. Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica 2007 Oct; 92(10): 1411–4
Dimopoulos MA, Richardson P, Schlag R, et al. Bortezomib-Melphalan-Prednisone (VMP) in newly diagnosed multiple myeloma patients with impaired renal function: cohort analysis of the phase III VISTA study [abstract no. 1727]. Blood 2008; 112(11): 608
European Medicines Agency. Velcade® — summary of product characteristics. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/velcade/H-539-PI-en.pdf [Accessed 2009 Apr 8]
Ogawa Y, Tobinai K, Ogura M, et al. Phase I and II pharmacokinetic and pharmacodynamic study of the proteasome inhibitor bortezomib in Japanese patients with relapsed or refractory multiple myeloma. Cancer Sci 2008; 99(1): 140–4
Pekol T, Daniels JS, Labutti J, et al. Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos 2005 Jun; 33(6): 771–7
Quinn DI, Nemunaitis J, Fuloria J, et al. Open-label study to assess effect of omeprazole on pharmacokinetics of bortezomib in subjects with advanced solid tumors, NHL, or multiple myeloma [abstract no. 13000]. J Clin Oncol 2007; 25(18S): 608S
Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 2004 Oct; 127(2): 165–72
Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003 Jun 26; 348(26): 2609–17
Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352(24): 2487–98
Mikhael JR, Belch AR, Prince HM, et al. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma; results of a global phase 3b expanded access program. Br J Haematol 2009 Jan; 144(2): 169–75
Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase 111 study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol 2007 Sep 1; 25(25): 3892–901
Palumbo A, Bringhen S, Rossi D, et al. A prospective, randomized, phase III study of bortezomib, melphalan, prednisone and thalidomide (VMPT) versus bortezomib, melphalan and prednisone (VMP) in elderly newly diagnosed myeloma patients [abstract no. 652]. Blood 2008; 112(11): 243. Plus oral presentation at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008 Aug 28; 359(9): 906–17
Cavo M, Tacchetti P, Patriarca P, et al. Superior complete response rate and progression-free survival after autologous transplantation with up-front velcade-thalidomide-dexamethasone compared with thalidomide-dexamethasone in newly diagnosed multiple myeloma [abstract no. 158]. Blood 2008; 112(11): 65. Plus oral presentation at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Harousseau JL, Mathiot C, Attal M, et al. VELCADE/dexamethasone (Vel/D) versus VAD as induction prior to autologous stem cell transplantation in previously untreated multiple myeloma: updated results from IFM 2005/01 trial [abstract no. 450]. Blood 2007; 110(11): 139a
Harousseau JL, Mathiot C, Attal M, et al. VELCADE/dexamethasone versus VAD as induction prior to ASCT in previously untreated multiple myeloma: updated results from IFM 2005/01 trial. Oral presentation at a Joint Annual Meeting of the American Society of Hematology-American Society of Clinical Oncology symposium held at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Sonneveld P, van der Holt B, Schmidt-Wolf IGH, et al. First analysis of HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, adriamycine, dexamethasone (PAD) vs VAD as induction treatment prior to high dose melphalan (HDM) in patients with newly diagnosed multiple myeloma (MM) [abstract no. 653]. Blood 2008; 112(11): 243–244. Plus oral presentation at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Blade J, Samson D, Reece D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoetic stem cell transplantation. Br J Haematolog 1998; 102: 115–23
Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20(9): 1467–73
Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumor malignancies. Clin Cancer Res 2002; 8(8): 2505–11
Richardson PG, Barlogie B, Berenson J, et al. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma: final time-to-event results from the SUMMIT trial. Cancer 2006 Mar 15; 106(6): 1316–9
Jagannath S, Barlogie B, Berenson JR, et al. Updated survival analyses after prolonged follow-up of the phase 2, multi-center CREST study of bortezomib in relapsed or refractory multiple myeloma. Br J Haematol 2008; 143(4): 537–40
Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 2007 Nov 15; 110(10): 3557–60
European Medicines Agency. European Public Assessment Report: initial scientific discussion of the approval of Velcade® [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/velcade/166104en6.pdf [Accessed 2008 Nov 18]
San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia 2008 Apr 1; 22(4): 842–9
Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol 2008 Jun; 19(6): 1160–5
Chanan-Khan AA, Padmanabhan S, Miller KC, et al. Final results of a phase II study of bortezomib (Velcade) in combination with liposomal doxorubicin (Doxil) and thalidomide demonstrate a sustained high response rates in patients with relapsed or refractory multiple myeloma [abstract no. 3539]. Blood 2006 Nov; 108(11): 1010a
Berenson JR, Yang HH, Sadler K, et al. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol 2006 Feb 20; 24(6): 937–44
Kim Y-K, Lee J-J, Sohn S-K, et al. Clinical efficacy of VEL-CTD (bortezomib, cyclophosphamide, thalidomide, and dexamethasone) regimen in patients with relapsed or refractory multiple myeloma: a phase II study [abstract no. 3693]. Blood 2008; 112(11): 1265. Plus poster presented at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Kropff M, Bisping G, Schuck E, et al. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol 2007 Aug; 138(3): 330–7
Palumbo A, Ambrosini MT, Benevolo G, et al. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood 2007 Apr 1; 109(7): 2767–72
Popat R, Oakervee H, Williams C, et al. Bortezomib, low-dose intravenous melphalan, and dexamethasone for patients with relapsed multiple myeloma. Br J Haematology 2009 Mar; 144(6): 887–94
Reece DE, Rodriguez GP, Chen C, et al. Phase I-II trial of bortezomib plus oral cyclophosphamide and prednisone in relapsed and refractory multiple myeloma. J Clin Oncol 2008 Oct 10; 26(29): 4777–83
Richardson P, Jagannath S, Jakubowiak A, et al. Lenali-domide, bortezomib, and dexamethasone in patients with relapsed or relapsed/refractory multiple myeloma (MM): encouraging response rates and tolerability with correlation of outcome and adverse cytogenetics in a phase II study [abstract no. 1742]. Blood 2008; 112(11): 614–5. Plus poster presented at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Terpos E, Kastritis E, Roussou M, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia 2008 Dec; 22(12): 2247–56
Richardson P, Wolf J, Jakubowiak A, et al. Phase I/II results of a multicenter trial of perifosine (KRX-0401) + bortezomib in patients with relapsed or refractory multiple myeloma who were previously relapsed from or refractory to bortezomib [abstract no. 870]. Blood 2008; 112(11): 321–2
Richardson P, Chanan-Khan AA, Lonial S, et al. Tane-spimycin (T) + bortezomib (BZ) in multiple myeloma (MM): confirmation of the recommended dose using a novel formulation [abstract no. 1165]. Blood 2007; 110(11): 353a
Weber D, Badros AZ, Jagannath S. Vorinostat plus bortezomib for the treatment of relapsed/refractory multiple myeloma: early clinical experience [abstract no. 871]. Blood 2008; 112(11): 322
Berenson JR, Matous J, Swift RA, et al. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res 2007 Mar 15; 13(6): 1762–8
Berenson JR, Yellin O, Patel R, et al. A phase I study of samarium lexidronam/bortezomib combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res 2009; 15(3): 1069–75
Rossi J-F, Manges RF, Sutherland HJ, et al. Preliminary results of CNTO 328, an anti-interleukin-6 monoclonal antibody, in combination with bortezomib in the treatment of relapsed or refractory multiple myeloma [abstract no. 867]. Blood 2008; 112(11): 320
Mateos MV, Hernández JM, Hernández MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood 2006 Oct 1; 108(7): 2165–72
Mateos MV, Hernández JM, Hernández MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: updated time-to-events results and prognostic factors for time to progression. Haematologica 2008 Apr; 93(4): 560–5
San Miguel JF, Schlag R, Khuageva NK, et al. Updated follow-up and results of subsequent therapy in the phase III VISTA trial: bortezomib plus melphalan-prednisone versus melphalan-prednisone in newly diagnosed multiple myeloma [abstract no. 650]. Blood 2008; 112(11): 242. Plus oral presentation at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Bensinger W, Jagannath S, Vescio R, et al. A phase II study of bortezomib (Velcade®), cyclophosphamide (Cytoxan®), thalidomide (Thalomid®) and dexamethasone as first-line therapy for multiple myeloma [abstract no. 94]. Blood 2008; 112(11): 42. Plus oral presentation at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Harousseau JL, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to auto-logous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica 2006 Nov; 91(11): 1498–505
Knop S, Liebisch P, Wandt H, et al. Bortezomib, intravenous cyclophosphamide and dexamethasone (VelCD) for previously untreated multiple myeloma: an interim analysis of the German DSMM trial [abstract no. 2776]. Blood 2008; 112(11): 958
Kumar S, Flinn IW, Noga SJ, et al. Safety and efficacy of novel combination therapy with bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in newly diagnosed multiple myeloma: initial results of a phase I/II multicenter EVOLUTION study [abstract no. 93]. Blood 2008; 112(11): 41–2
Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol 2005 Jun; 129(6): 755–62
Palumbo A, Falco P, Gay F, et al. Bortezomib-doxor-ubicin-dexamethasone as induction prior to reduced intensity autologous transplantation followed by lenalidomide as consolidation/maintenance in elderly untreated myeloma patients [abstract no. 159]. Blood 2008; 112(11): 65. Plus oral presentation at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Popat R, Oakervee HE, Hallam S, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. Br J Haematol 2008 May; 141(4): 512–6
Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. Epub 2009 Feb 19
Richardson P, Lonial S, Jakubowiak A. Lenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma: encouraging efficacy in high risk groups with updated results of a phase I/II study [abstract no. 92]. Blood 2008; 112(11): 41. Plus oral presentation at the 50th Annual Meeting of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Kaufman JL, Gleason C, Heffner L, et al. Bortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myeloma [abstract no. 3605]. Blood 2007; 110: 1055a
Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bor-tezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol 2009; 144(6): 895–903
Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with borte-zomib for relapsed, refractory multiple myeloma. Blood 2005 Dec 1; 106(12): 3777–84
Lonial S, Richardson PG, San Miguel J, et al. Characterisation of haematological profiles and low risk of thromboembolic events with bortezomib in patients with relapsed multiple myeloma. Br J Haematol 2008 Oct; 143(2): 222–9
Richardson P, Schlag R, Khuageva NK, et al. Erythropoiesis-stimulating agents do not adversely affect long-term outcomes nor increase the risk of thromboembolic events in multiple myeloma patients treated in the phase III VISTA trial [abstract no. 1741]. Blood 2008; 112(11): 614
Kane RC, Farrell AT, Sridhara R, et al. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 2006 May 15; 12(10): 2955–60
Plosker GL. Pegylated liposomal doxorubicin: a review of its use in the treatment of relapsed or refractory multiple myeloma. Drugs 2008; 68(17): 2535–51
van de Velde HJ, Liu X, Chen G. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica 2007; 92(10): 1399–406
Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet 2006 Mar 11; 367(9513): 825–31
Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone alone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 2007; 370(9594): 1209–18
US National Institutes of Heath. Study of Velcade® and bone formation in patients with relapsed/refractory multiple myeloma NCT00128921 [online]. Available from URL: http://clinicaltrials.gov [Accessed 2009 Feb 8]
Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008; 112(5): 1593–9
Hrusovsky I, Emmerich B, von Rohr A, et al. Bortezomib retreatment in relapsed multiple myeloma (MM): results from a binational, multicenter retrospective survey [abstract no. 2775]. Blood 2008; 112(11): 957–958
Wolf J, Richardson PG, Schuster M, et al. Utility of bortezomib retreatment in relapsed or refractory multiple myeloma patients: a multicenter case series. Clin Adv Hematol Oncol 2008; 6(10): 755–60
Conner TM, Doan QD, Walters IB, et al. An observational, retrospective analysis of retreatment with bortezomib for multiple myeloma. Clin Lymphoma Myeloma 2008 Jun; 8(3): 140–5
Jaggannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia 2007; 21(1): 151–7
Roussou M, Kastritis E, Migkou M, et al. Treatment of patients with multiple myeloma complicated by renal failure with bortezomib-based regimens. Leuk Lymphoma 2008 May; 49(5): 890–5
Jagannath S, Barlogie B, Berenson JR, et al. Bortezomib in recurrent and/or refractory multiple myeloma; initial clinical experience in patients with impaired renal function. Cancer 2005; 103(6): 1195–200
U.S. National Institutes of Health. Clinical trials of bortezomib [online]. Available from URL: http://clinicaltrials.gov [Accessed 2009 Mar 3]
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J.R. Berenson, Institute for Myeloma and Bone Cancer Research Hematology/Oncology, West Hollywood, California, USA; S.K. Kumar, Division of Hematology, Mayo Clinic, Rochester, Minnesota, USA; S. Lonial, Winship Cancer Institute Emory, University School of Medicine, Emory University Atlanta, Atlanta, Georgia, USA; A Palumbo, University of Torino, Turin, Italy; S.R. Shah, School of Pharmacy, Texas Tech University Health Sciences Center, Dallas, Texas, USA; E. Terpos, Department of Clinical Therapeutics, Athens School of Medicine, Alexandra General Hospital, Athens, Greece.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘bortezomib’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘bortezomib’ and ‘multiple myeloma’. Searches were last updated 24 April 2009.
Selection: Studies in patients with multiple myeloma who received bortezomib. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Bortezomib, multiple myeloma, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Curran, M.P., McKeage, K. Bortezomib. Drugs 69, 859–888 (2009). https://doi.org/10.2165/00003495-200969070-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200969070-00006